ANSWER:
Neon has three isotopes Ne–20, Ne–21 & Ne–22, having percentage abundance of 90.92%, 0.26% & 8.82% respectively. When we calculate average of all these isotopic masses in proportion to their natural abundances, a fractional value of 20.18 amu is obtained that is called the average atomic mass of Neon.
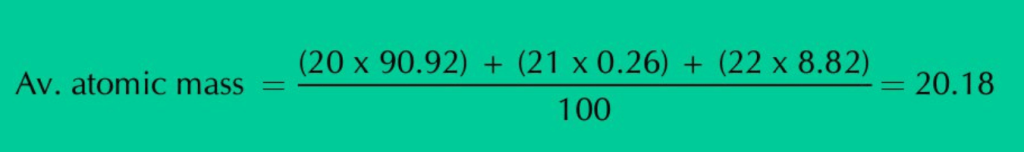
So, 20.18 amu is not the mass of any individual Neon atom. Rather, it is the average or relative atomic mass of Neon.
(Ref. An Insight Into Objective Chemsitry-11, Ch-01, Q. No. 27)

