BASIC CONCEPTS
Here is the complete solution to textbook exercise of chapter-01, Chemistry-11:
Q.1: Select the most suitable answer from the given ones in each question.
(i) Isotopes differ in:
(a) properties which depend upon mass
(b) arrangement of electrons in orbitals
(c) chemical properties
(d) the extent to which they may be affected in electromagnetic field.
Ans: (a) properties which depend upon mass
EXPLANATION: Isotopes have same atomic number but different atomic masses. So, they differ in those properties which depend upon masses such as density, diffusion etc.
(ii) Select the most suitable answer from the given ones in each question:
(a) Isotopes with even atomic masses are comparatively abundant.
(b) Isotopes with odd atomic masses are comparatively abundant.
(c) Isotopes with even atomic masses and even atomic numbers are comparatively abundant.
(d) Isotopes with even atomic masses and odd atomic numbers are comparatively abundant.
Ans: (c) Isotopes with even atomic masses and even atomic numbers are comparatively abundant.
EXPLANATION: The elements of even atomic number usually have larger number of isotopes and isotopes whose mass numbers are multiples of four are particularly abundant. For example, l6O, 24Mg, 28Si, 40Ca & 56Fe form nearly 50% of the earth’s crust. Out of 280 isotopes that occur in nature, 154 have even mass number and even atomic number.
(iii) Many elements have fractional atomic masses. This is because:
(a) the mass of the atom is itself fractional.
(b) atomic masses are average masses of isobars.
(c) atomic masses are average masses of isotopes.
(d) atomic masses are average masses of isotopes proportional to their relative abundance.
Ans: (d) atomic masses are average masses of isotopes proportional to their relative abundance.
EXPLANATION: The atomic mass of an element is not the mass of an individual atom of that element. Rather, it is the average of all its isotopic masses relative to their natural abundances. That’s why, its value is in fractions. Moreover, the presence of various subatomic particles in the atom also makes the relative atomic mass of an element fractional.
(iv) The mass of one mole of electrons is:
(a) 1.008 mg
(b) 0.55 mg
(c) 0.184 mg
(d)1.673 mg
Ans: (b) 0.55 mg
EXPLANATION: One mole of electrons means 6.02 ✕ 1023 electrons. The mass of one electron is about 9.1095 ✕ 10-31 kg. So, by multiplying the number of electrons in one mole with the mass of one electron, and then changing the unit of kilogram into milligrams, we get the answer 0.55 mg.
(v) 27 g of Al will react completely with how much mass of O2 to produce Al2O3:
(a) 8 g of oxygen
(b) 16 g of oxygen
(c) 32 g of oxygen
(d) 24 g of oxygen
Ans: (d) 24 g of Oxygen
EXPLANATION: According to the equation 4Al + 3O2 ⟶ 2Al2O3, 4 moles (108 g) of Al react with 3 moles (96 g) of O2. So, 27 g of Al will react with 24 g of O2 (96/108 ✕ 27 = 24 g).
(vi) The number of moles of CO2 which contain 8.0 g of oxygen:
(a) 0.25
(b) 0.50
(c) 1.0
(d)1.50
Ans: (a) 0.25
EXPLANATION: One mole of CO2 (44 g) contains one mole (32 g) of O2. So, 8 grams of oxygen will be present in 0.25 moles of CO2. (Formula: 1/32 ✕ 8 = 0.25 moles of CO2.
(vii) The largest number of molecules are present in:
(a) 3.6 g of H2O
(b) 4.8 g of C2H5OH
(c) 2.8 g of CO
(d) 5.4 g of N2O5
Ans: (a) 3.6 g of H2O
EXPLANATION: The greater the number of moles is, the greater is the number of molecules of the compound and vice versa. First, calculate the number of moles of each compound and then multiplying with Avogadro’s number (NA= 6.02 x 1023), we reach at the conclusion that 3.6 grams of H2O has greater number of molecules. (Formula: No. of molecules = Mass/Molar mass ✕ Avogadro’s Number OR N=m/M ✕ NA)
(viii) One mole of SO2 contains:
(a) 6.02 x 1023 atoms of Oxygen
(b) 18.1 x 1023 molecules of SO2
(c) 6.02 x 1023 atoms of Sulphur
(d) 4 gram atoms of SO2
Ans: (c) 6.02 x 1023 atoms of Sulphur
EXPLANATION: One mole of SO2 contains one Avogadro’s number (6.02 x 1023) molecules of SO2, one Avogadro’s number atoms of S and two Avogadro’s number atoms of O2. So, according to this information, option (c) is correct.
(ix) The volume occupied by 1.4 g of N2 at S.T.P is:
(a) 2.24 dm3
(b) 22.4 dm3
(c) 1.12 dm3
(d) 112 cm3
Ans: 1.12 dm3
EXPLANATION: One mole of N2 (28 g) at STP contains volume 22.414 dm3. So, 1.4 g of N2 will occupy volume 1.12 dm3. (Formula: 22.414 dm3/28 g ✕ 1.4 g = 1.12 dm3)
(x) A limiting reactant is the one which:
(a) is taken in lesser quantity in grams as compared to other reactants.
(b) is taken in lesser quantity in volume as compared to the other reactants.
(c) gives the maximum amount of the product which is required.
(d) gives the minimum amount of the product under consideration.
Ans: (d) gives the minimum amount of the product under consideration.
EXPLANATION: Limiting reactant is present in less amount as required according to balanced chemical equation. So, it is consumed earlier in the reaction, causes to stop the reaction, and ultimately gives minimum amount of the product under consideration.
Q.02: Fill in the blanks:
(i) The unit of relative atomic mass is_________________.
(amu)
(ii) The exact masses of isotopes can be determined by________________ spectrograph.
(Aston’s mass)
(iii) The phenomenon of isotopy was first discovered by ____________ .
(Soddy)
(iv) Empirical formula can be determined by combustion analysis for those compounds which have ________ and _________ in them.
(Carbon & Hydrogen)
(v) A limiting reagent is that which controls the quantities of __________ .
(Products)
(vi) 1 mole of glucose has ____________ atoms of carbon, ______________ of oxygen and ______________ of H.
(3.612 ✕ 1024, 3.612 ✕ 1024, 7.224 ✕ 1024)
(vii) 4 g of CH4 at 0°C and 1 atm pressure has _____________ molecules of CH4.
(1.505 ✕ 1023)
(viii) Stoichiometric calculations can be performed only when __________ is obeyed.
(Law of conservation of mass & Law of definite proportions)
Q.03: Indicate true or false as the case may be:
(i) Neon has three isotopes and the fourth one with atomic mass 20.18 amu.
Ans: (False)
Neon has three isotopes, there is no fourth one with atomic number 20.18 amu. Actually, 20.18 amu is the relative atomic mass of Neon which is the average mass of all its three isotopes, relative to their natural abundance.
(ii) Empirical formula gives the information about the total number of atoms present in the molecule.
Ans: (False)
Empirical formula gives the simple whole number ratio between the atoms of elements in a compound.
(iii) During combustion analysis Mg(ClO4)2 is employed to absorb water vapours.
Ans: (True)
(iv) Molecular formula is the integral multiple of empirical formula and integral multiple can never be unity.
Ans: (False)
Molecular formula is the integral multiple of empirical formula and integral multiple can be unity for several molecules which have same empirical and molecular formulas. However, it never be zero.
(v) The number of atoms in 1.79 g of gold and 0.023 g of sodium are equal.
Ans: (True)
(vi) The number of electrons in the molecules of CO and N2 are 14 each, so 1 g of each gas will have same number of electrons.
Ans: (True)
(vii) Avogadro’s hypothesis is applicable to all types of gases i.e., ideal and non-ideal.
Ans: (False)
Avogadro’s hypothesis is applicable only to ideal gases because non-ideal gases don’t obey gas laws.
(viii) Actual yield of a chemical reaction may be greater than the theoretical yield.
Ans: (False)
Actual yield can never be greater than theoretical. Rather, it is mostly smaller than theoretical yield because some of the product may be lost during the preparation or separation processes.
Q.4: What are ions? Under what conditions are they produced?
Ans:
IONS: “Those species which carry either positive or negative charge are called ions.”
Examples: K+, Mg2+, Al3+, Cl–, O2-, CO32- etc.
Conditions for the Formation of Ions:
Positive ions or cations are formed when an atom loses one or more electrons. This requires energy, which is used to release the electron from the attraction of the nucleus and to bring it out of the atom. e.g.,
M ➜ M+ + e– ΔH = Positive
Negative ions or anions are formed when an atom gains one or more electrons. During this process, energy is usually released due to the attraction of the nucleus to the incoming electron. e.g.,
B + e– ➜ B– ΔH = Negative
Q.05: (a) What are isotopes? How do you deduce the fractional atomic masses of elements from the relative isotopic abundance? Give two examples in support of your answer.
Ans:
ISOTOPES: “The atoms of the same element having same atomic number but different atomic masses are called isotopes.”
Isotopes have same position in the periodic table. They have same chemical but different physical properties.
Fractional Atomic Masses from Relative Isotopic Abundance:
Fractional or average atomic mass of an element can be calculated by multiplying the relative abundance with isotopic mass, and then dividing their sum by 100. For example:
(1) Neon has three isotopes, Ne-20, ne-21 & Ne-22 present in the percentage of 90.92%, 0.26% & 8.825 respectively. Its average atomic mass can be calculated as:
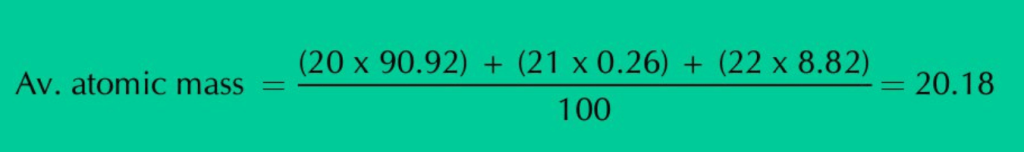
(2) Similarly, the average atomic mass of silver can be calculated from its isotopic abundances as:
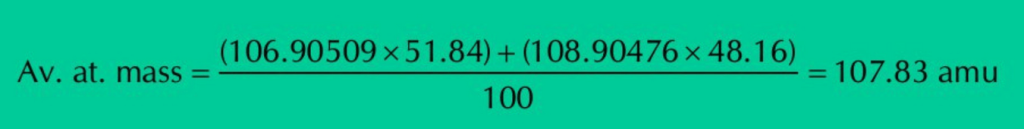
(b) How does a mass spectrograph show the relative abundance of isotopes of an element? What is the justification of two strong peaks in the mass spectrum for Bromine while for Iodine only one peak at 127 amu is indicated?
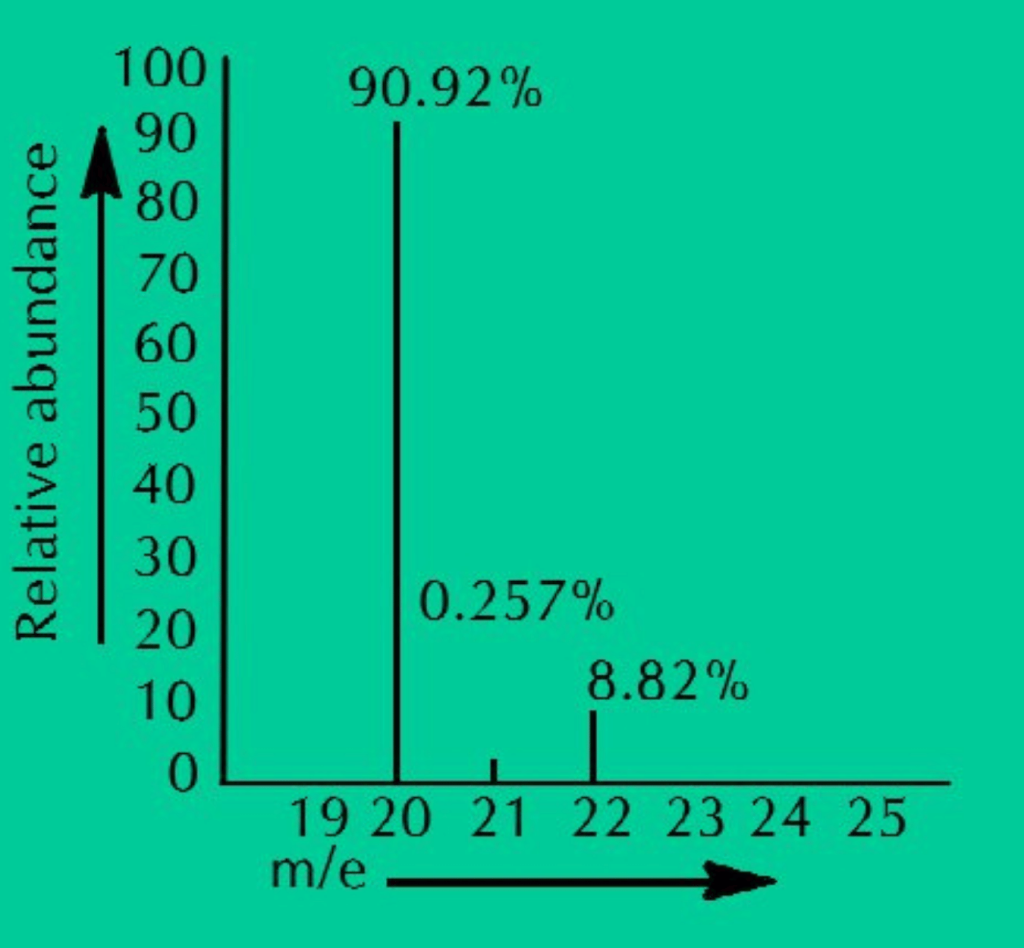
Ans: Mass spectrograph or mass spectrometer is used to determine the relative abundances and masses of various isotopes of an element. The gaseous sample of an element is ionized and passed through electric and magnetic fields which separates different isotopes on the bases of their masses. These isotopic ions are then made to fall on an ion collector. The ionic current produced is amplified and is fed to the recorder. The recorder makes a graph showing the relative abundance of isotopes plotted against the mass number.
(c) What is the justification of two strong peaks in the mass spectrum for Bromine while for Iodine only one peak at 127 amu is indicated?
Ans: In mass spectrum, the number of peaks represents the number of isotopes, while the heights of the peaks represent their natural abundance. Two equally strong peaks in the mass spectrum of bromine indicate that bromine has two isotopes which have almost equal abundance. While, only one peak for iodine at 127 amu indicates that iodine has only one isotope with atomic mass 127 amu.
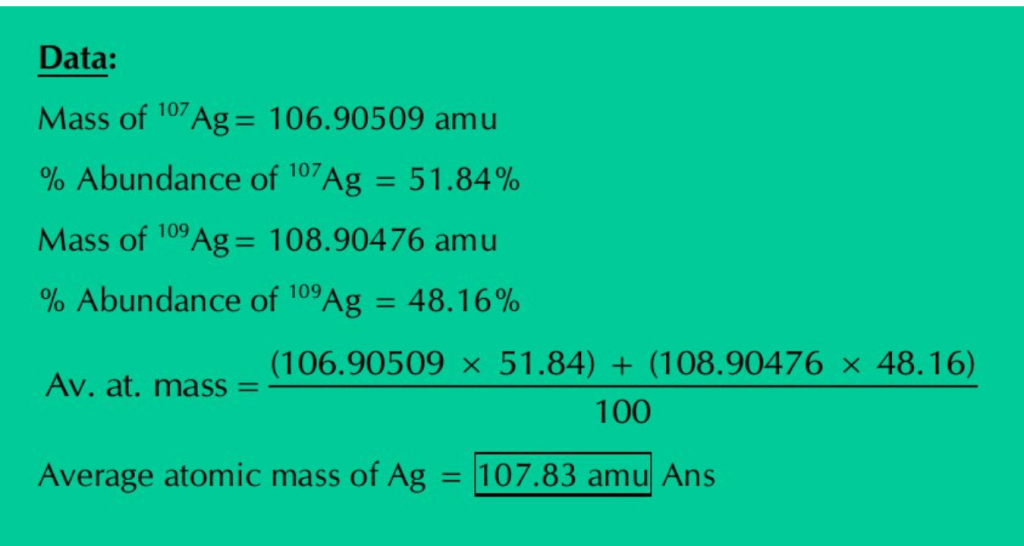
Q.6: Silver has atomic number 47 and has 16 known isotopes but two occur naturally i.e., Ag-107 & Ag-109. Given the following mass spectrometric data, calculate the average atomic mass of silver.
| Isotopes | Mass(amu) | % Abundance |
| 107Ag | 106.90509 | 51.84 |
| 109Ag | 108.90476 | 48.16 |
Solution:
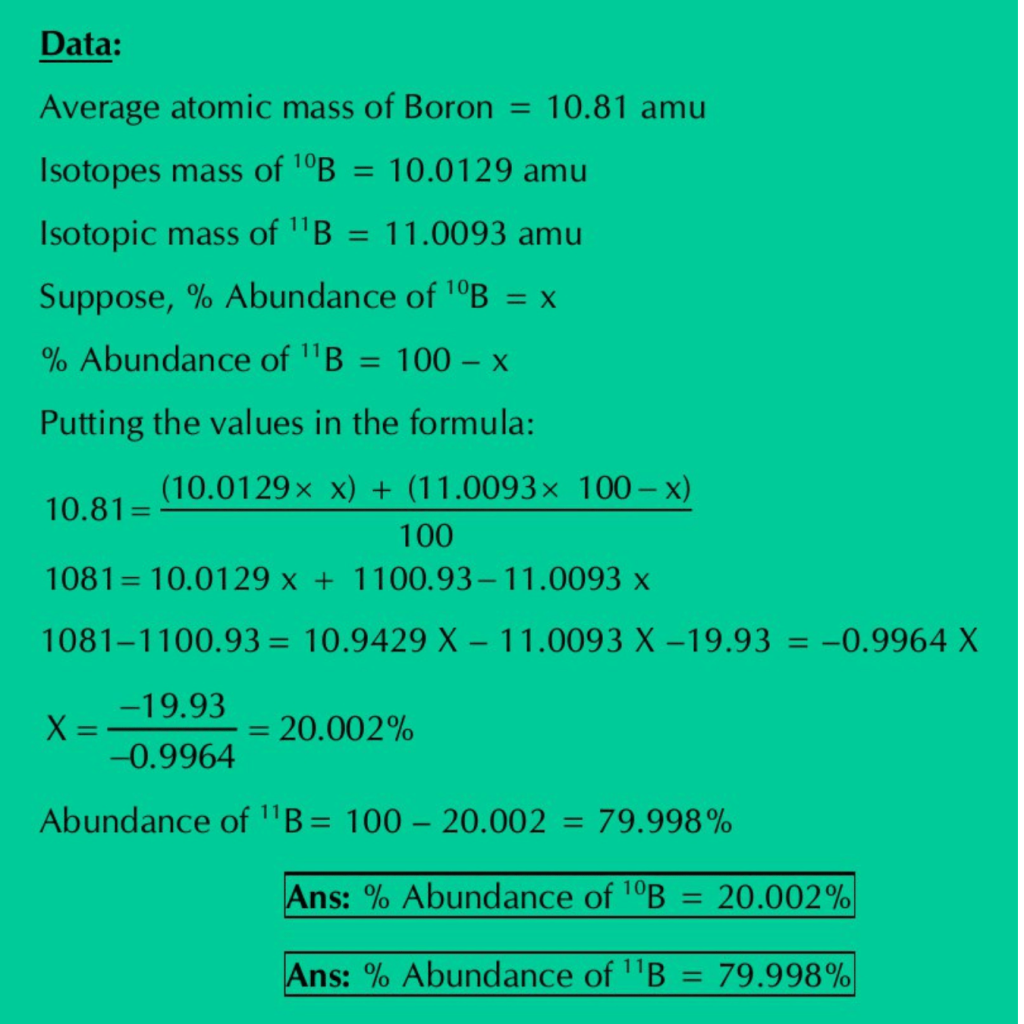
Q.07: Boron with atomic number 5 has two naturally occurring isotopes. Calculate the percentage abundance of 10B & 11B from the following information: Average at. mass of boron = 10.81 amu / Isotopic mass of 10B= 10.0129 amu / Isotopic mass of 11B= 11.0093 amu.
Solution:
Q.08: Define the following terms and give three examples of each: (i) Gram atom (ii) Gram molecular mass (iii) Gram formula (iv) Gram ion (v) Molar volume (vi) Avogadro’s number (vii) Stoichiometry (viii) Percentage yield.
Ans:
GRAM ATOM: “The atomic mass of an element expressed in grams is called one gram atom.” It is also called one gram mole or simply one mole of that element.

Examples:
1 gram atom of Hydrogen=1.008 g
1 gram atom of Carbon=12.000 g
GRAM MOLECULAR MASS: “The molecular mass of a substance expressed in grams is called one gram molecule or one gram mole or simply one mole of that substance.”

Examples:
1 gram molecule of water = 18 g
1 gram molecule of H2SO4 = 98 g
GRAM FORMULA: “The formula unit mass of an ionic compound expressed in grams is called one gram formula of the substance.”

Examples:
1 gram formula of NaCl = 58.5 g
1 gram formula of Na2CO3 = 106 g
GRAM ION: “The ionic mass of an ionic species expressed in grams is called one gram ion or one mole of ions.”

Examples:
1 gram ion of OH– =17 g
1 gram ion of SO42- = 96 g
MOLAR VOLUME: “One mole of any gas at standard temperature and pressure contains volume 22.414 dm3. This volume of 22.414 dm3 is called molar volume.” It is true when the gas is ideal.
Examples:
- 2.016 g of H2 = 1 mole of H2 = 6.02×1023 molecules of H2 = 22.414 dm3 of H2 at S.T.P.
- 16 g of CH4 =1 mole of CH4 = 6.02×1023 molecules of CH4 =22.414 dm3 of CH4 at S.T.P
AVOGADRO’S NUMBER “The number of atoms, molecules, formula units and ions in one gram atom of an element, one gram molecule of a compound, one gram formula of an ionic substance and one gram ion of an ionic specie respectively is equal to 6.02×1023. This number is called Avogadro’s number and is represented by NA.”
Examples:
- 1.008 g of H=1 mole of hydrogen=6.02×1023 atoms of H
- 18 g of H2O =1 mole of water = 6.02×1023 molecule of water
- 58.5 g of NaCl=1 mole of NaCl=6.02×1023 formula units of NaCl
- 96 g of = 1 mole of = 6.02×1023 ions
STOICHIOMETRY: “Stoichiometry is the branch of chemistry which tells us the quantitative relationship between reactants and products in a balanced chemical equation.”
Examples: Following types of relationship can be studied with the help of balanced chemical equation:
Mass–Mass Relationship: If we are given the mass of one substance, we can calculate the mass of the other substances involved in the chemical reaction.
Mass–Mole or Mole–Mass Relationship: If we are given the mass of one substance, we can calculate the moles of the other substances and vice versa.
Mass–Volume Relationship: If we are given the mass of one substance, we can calculate the volume of the other substances and vice versa.
Percentage Yield: The efficiency of a chemical reaction is calculated by comparing the actual and theoretical yields, in the form of percentage yield.
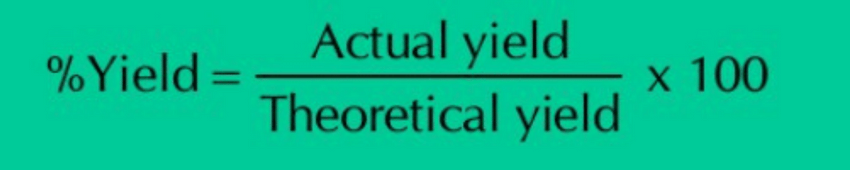
The greater is the percentage yield, the greater is the efficiency, and vice versa.
Q.09: Justify the following statements:
(a) 23 g of sodium ad 238 g of uranium have equal number of atoms in them.
Ans: 23 g of sodium and 238 g of uranium have equal number of atoms because these are the molar masses (atomic masses expressed in grams) of these elements, and we know that one mole of every element contains Avogadro’s number of atoms or 6.02×1023 atoms.
(b) Mg atom is twice heavier than that of carbon atom.
Ans: Mg atom is twice heavier as an atom of carbon because the molar mass of Mg is 24 g mol-1 while that of C is 12 g mol-1. It means Avogadro’s number of Mg atoms (6.02×1023) contain double mass as compared to equal number of C atoms. Comparing the masses and the number of both Mg and C atoms, it becomes clear that one Mg atom is twice heavier than one C atom.
(c) 180 g of glucose and 342 g of sucrose have the same number of molecules but different number of atoms present in them.
Ans: 180 g is equal to on mole of glucose, while 342 g is equal to one mole of sucrose. Since, one mole of a compound contains Avogadro’s number of molecules, so both glucose and sucrose have same number of molecules (i.e., 6.02×1023). On the other hand, number of atoms in both is different. This is because one molecule of glucose (C6H12O6) contains 24 atoms, while one molecule of sucrose (C12H22O11) contains 45 atoms. So, both have different number of atoms.
(d) 4.9 g of H2SO4 when completely ionized in water, have equal number of positive and negative charges but the number of positively charged ions are twice the number of negatively charged ions.
Ans: 4.9 g of H2SO4 when completely ionized in water, have equal number of positive and negative charges but the number of positively charged ions are twice the number of negatively charged ions because one H2SO4 molecule ionizes to give two H+ ions and one SO4– ion. So, the number of positive ions will be double as compared to the negative ions. On the other hand, number of opposite charges will be equal. This is because each H+ ion bears single positive charge while each SO4– ion bears double negative charge. So, double number of H+ ions will bear equal charge as compared to half number of SO4– ions.
(e) One mg of K2CrO4 has thrice the number of ions than the number of formula units when ionized in water.
Ans: K2CrO4 ionizes in water as:
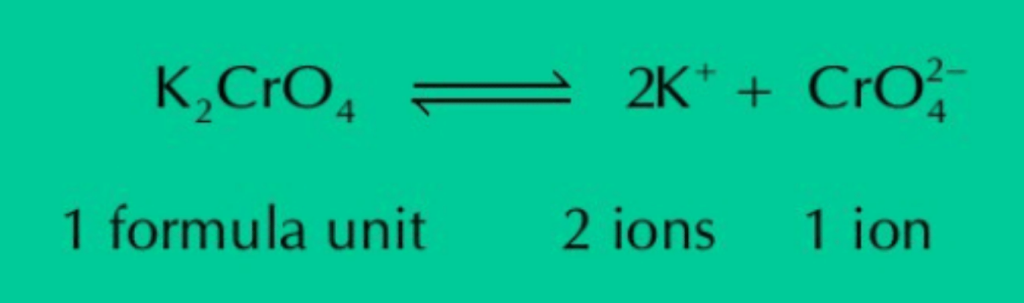
The equation shows that whatever the mass of K2CrO4 is, its one formula unit ionizes in water to give two K+ ions and one ion. Thus, total number of ions will always be thrice of the number of formula units.
(f) 2 grams of H2, 16 grams of CH4 and 44 g of CO2 occupy separately the volumes 22.414 dm3, although the sizes and masses of three gases are very different from each other.
Ans: 2 grams of H2, 16 grams of CH4 and 44 g of CO2 occupy separately the volumes 22.414 dm3, although the sizes and masses of three gases are very different from each other. This is because the volumes of gases depend upon the number of molecules, not upon the sizes or masses of molecules. This is due to the fact that the gas molecules far apart from each other, about 300 times than their diameters. So, the sizes or masses of molecules don’t affect each other due to large empty spaces between them.
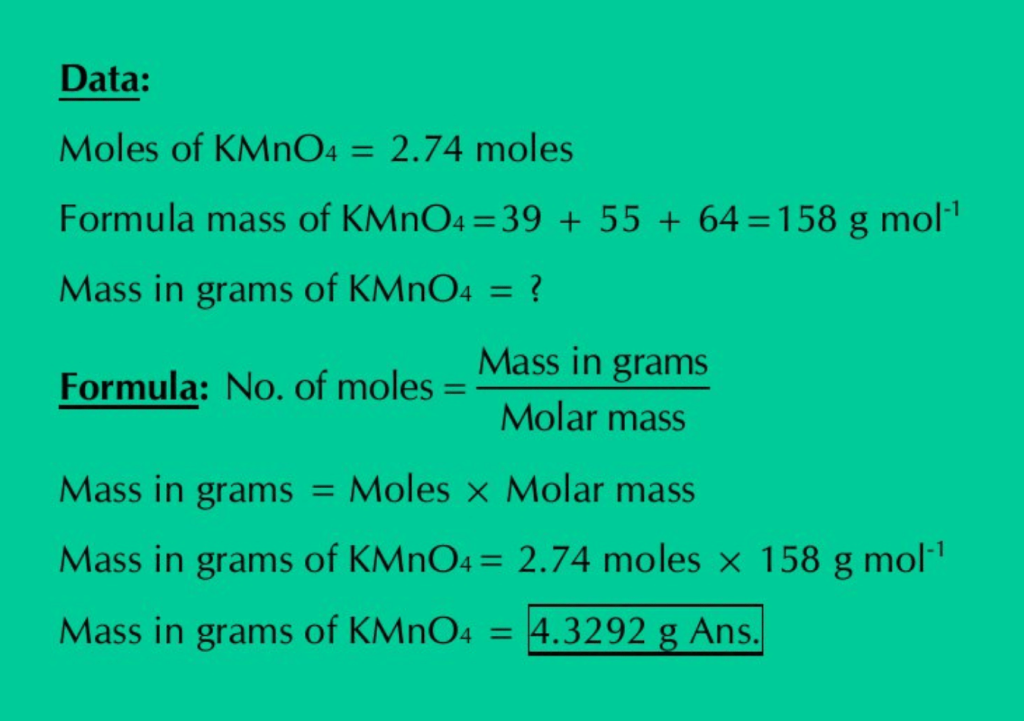
Q.10(a): Calculate the mass in grams of 2.74 moles of KMnO4.
Solution:
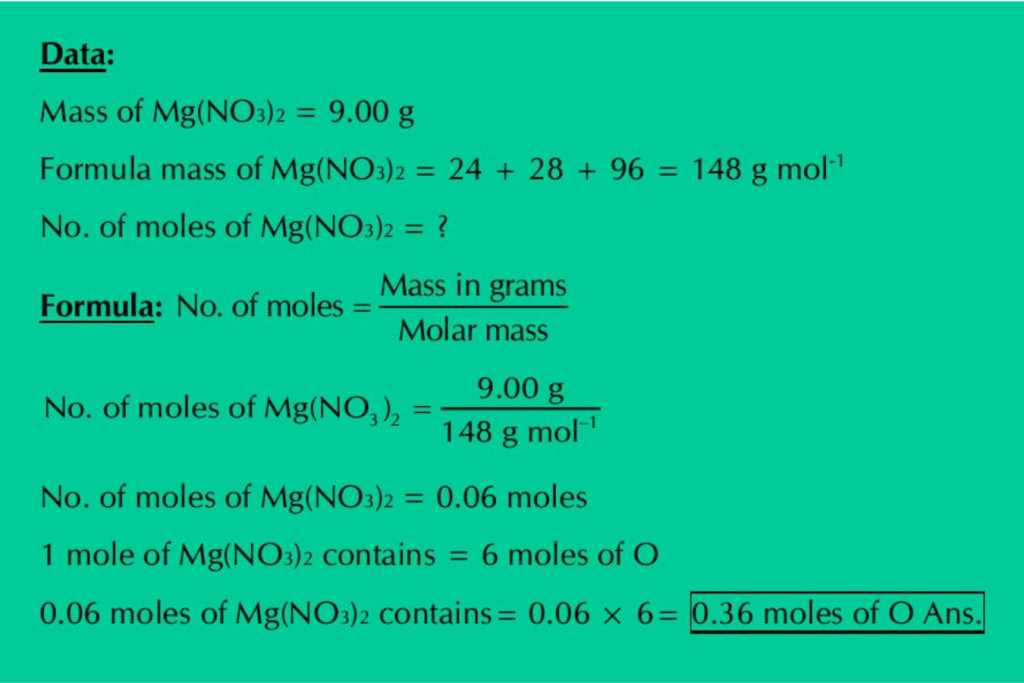
(b) Calculate the moles of O atoms in 9.00 g of Mg(NO3)2.
Solution:
(c) Calculate the number of O atoms in 10.037 g of CuSO4.5H2O.
Solution:

(d) Calculate the mass in kilograms of 2.6 × 1020 molecules of SO2.
Solution:
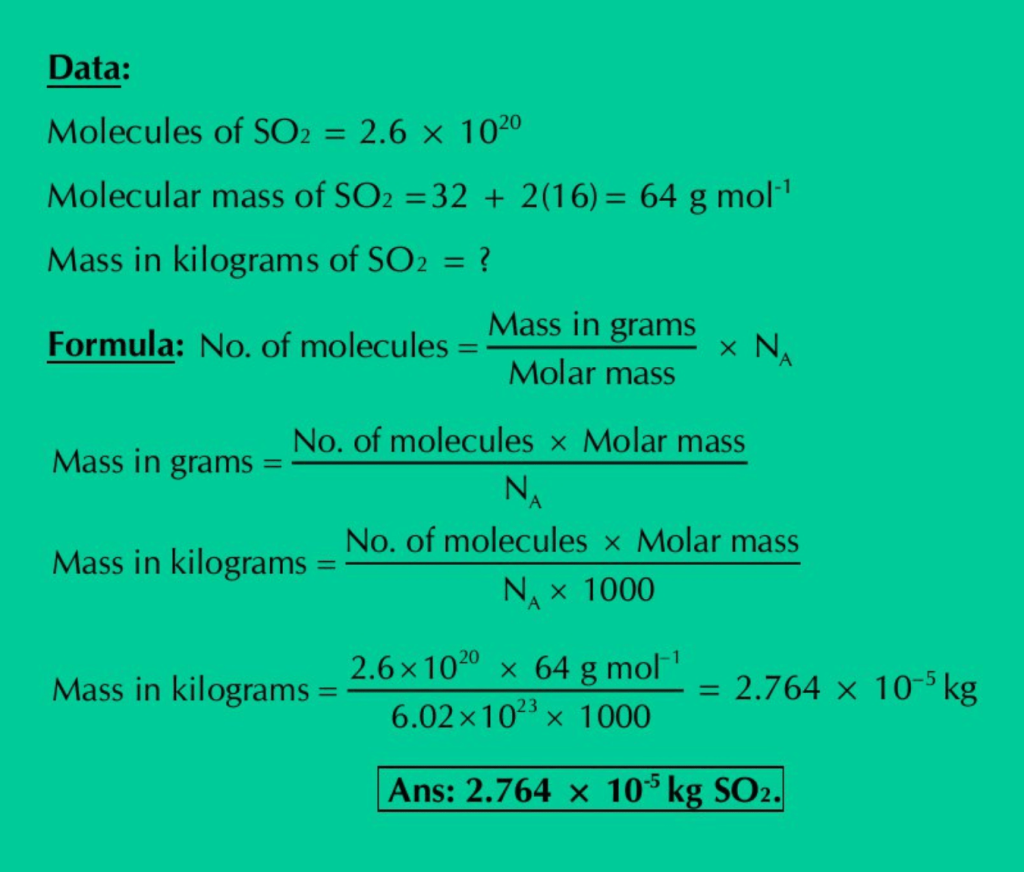
(e) Calculate the moles of Cl atoms in 0.822 g of C2H4Cl2.
Solution:
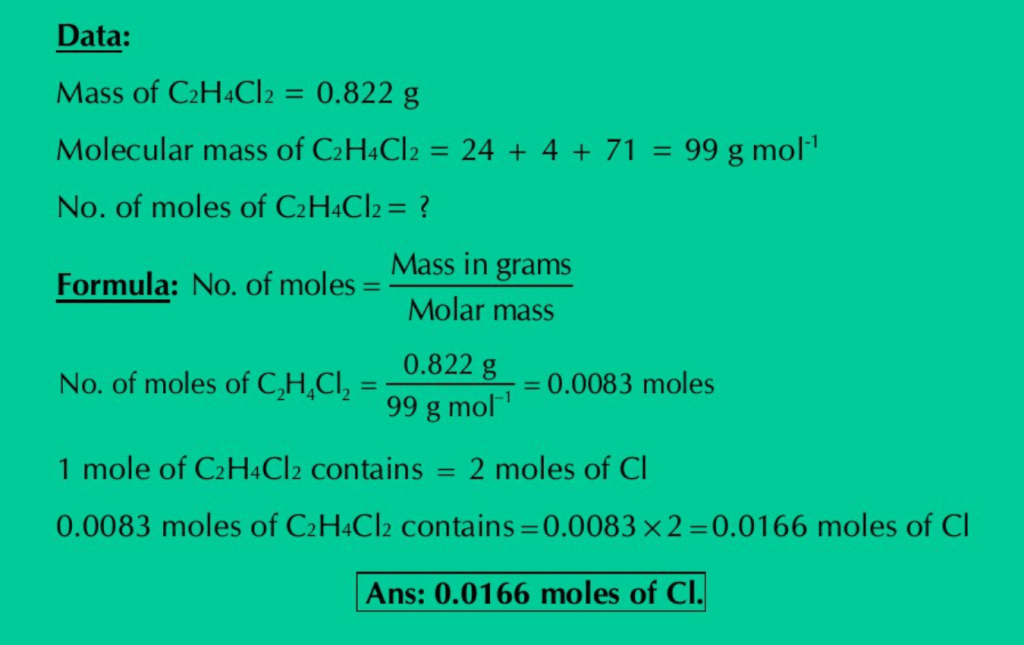
(f) Calculate the mass in grams of 5.136 moles of Ag2CO3.
Solution:

(g) Calculate the mass in grams of 2.78×1021 molecules of CrO2Cl2.
Solution:
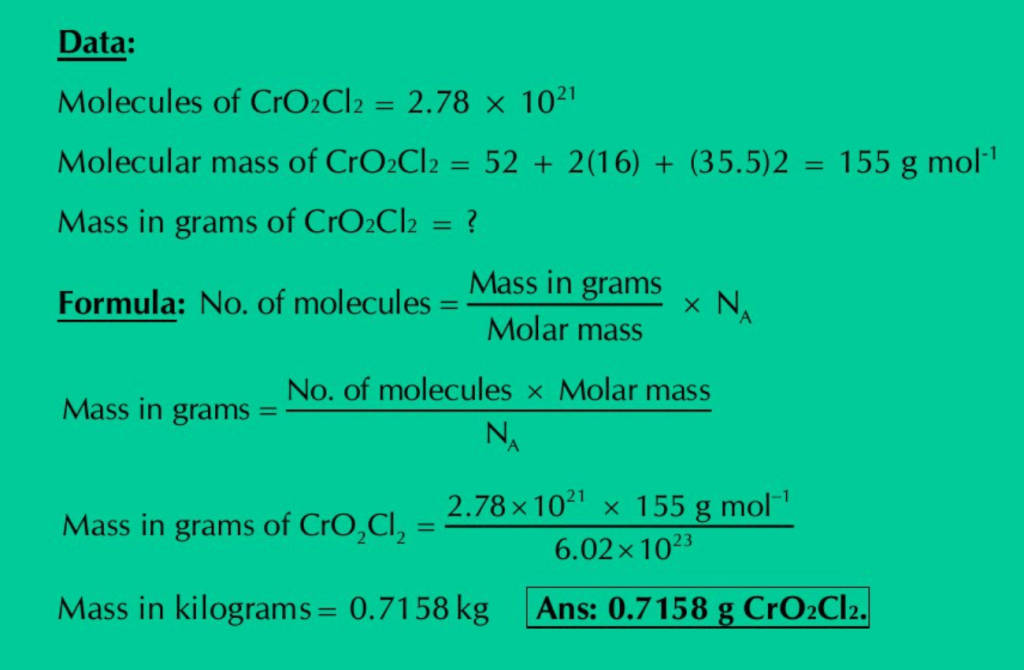
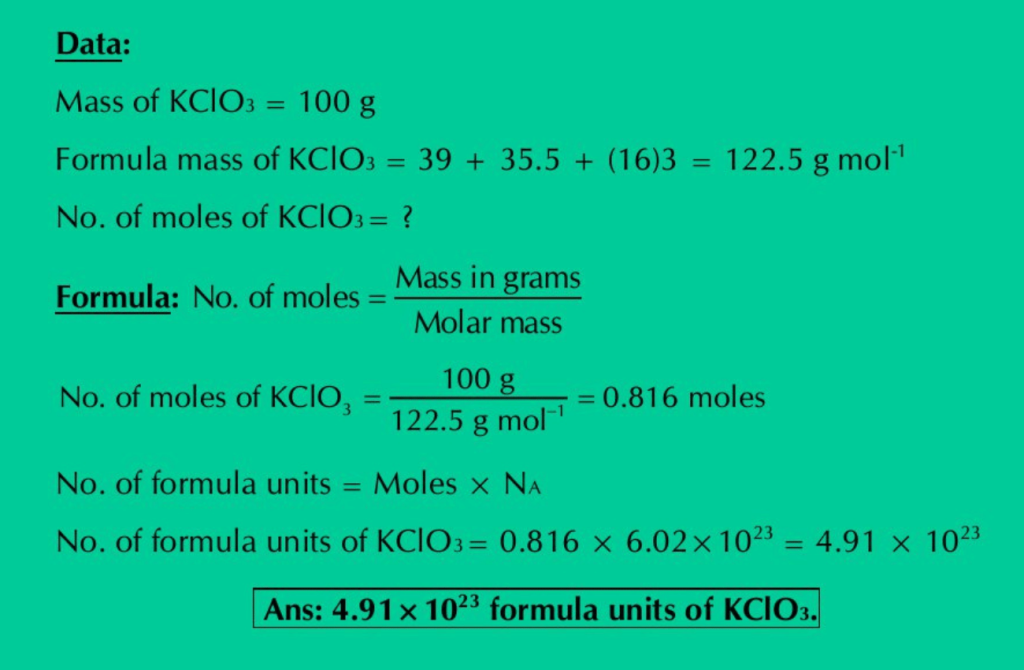
(h) Calculate the number of moles & formula units in 100 g of KClO3.
Solution:
(i) Calculate the number K+ ions, ions, Cl atoms & O atoms in (h).
Solution:

Q.11: Aspartame, the artificial sweetener, has a molecular formula of C14H18N2O5.
a) What is the mass of one mole of aspartame?
b) How many moles are present in 52 g of aspartame?
c) What is the mass in grams of 10.122 moles of aspartame?
d) How many hydrogen atoms are present in 2.43 g of aspartame?
Solution:

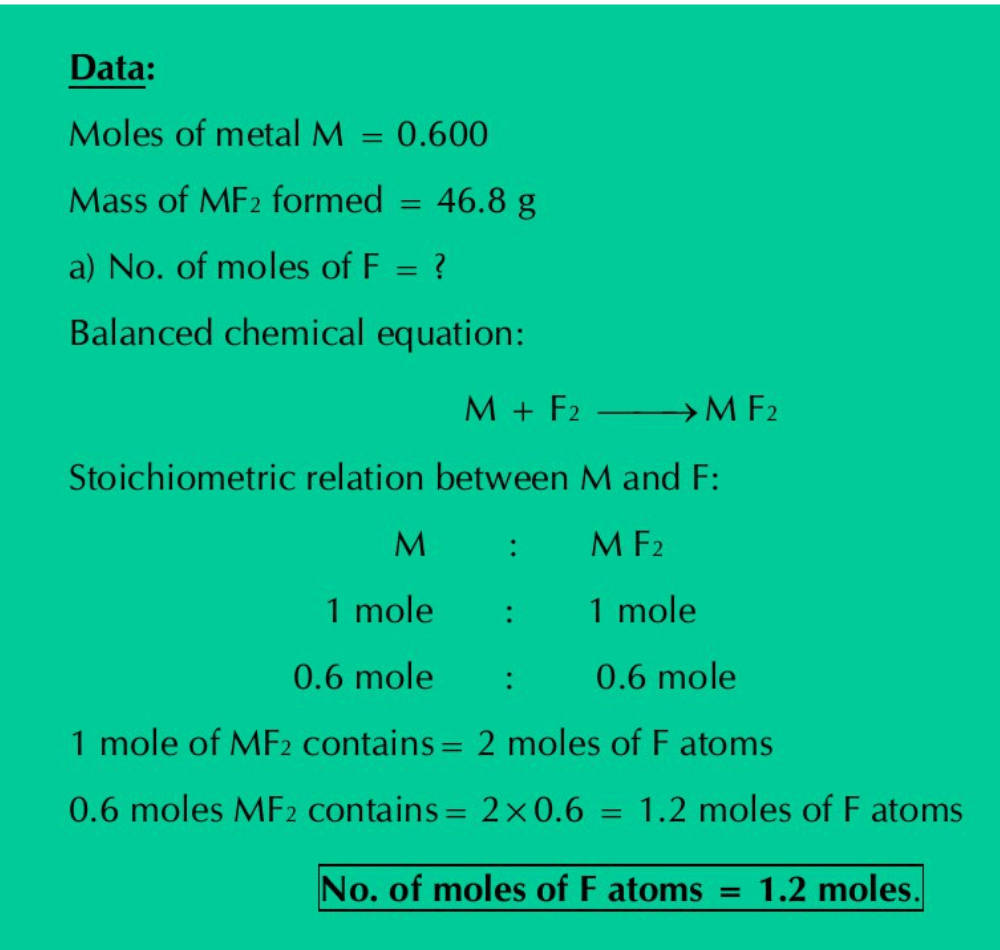
Q.12: A sample of 0.600 mole of metal M reacts completely with excess of fluorine to form 46.8 g of MF2.
How many moles of F are present in the sample of MF2 formed?
Which element is represented by the symbol M?
Solution:

Q.13: In each pair, choose the larger of the indicated quantity, or state if the samples are equal.
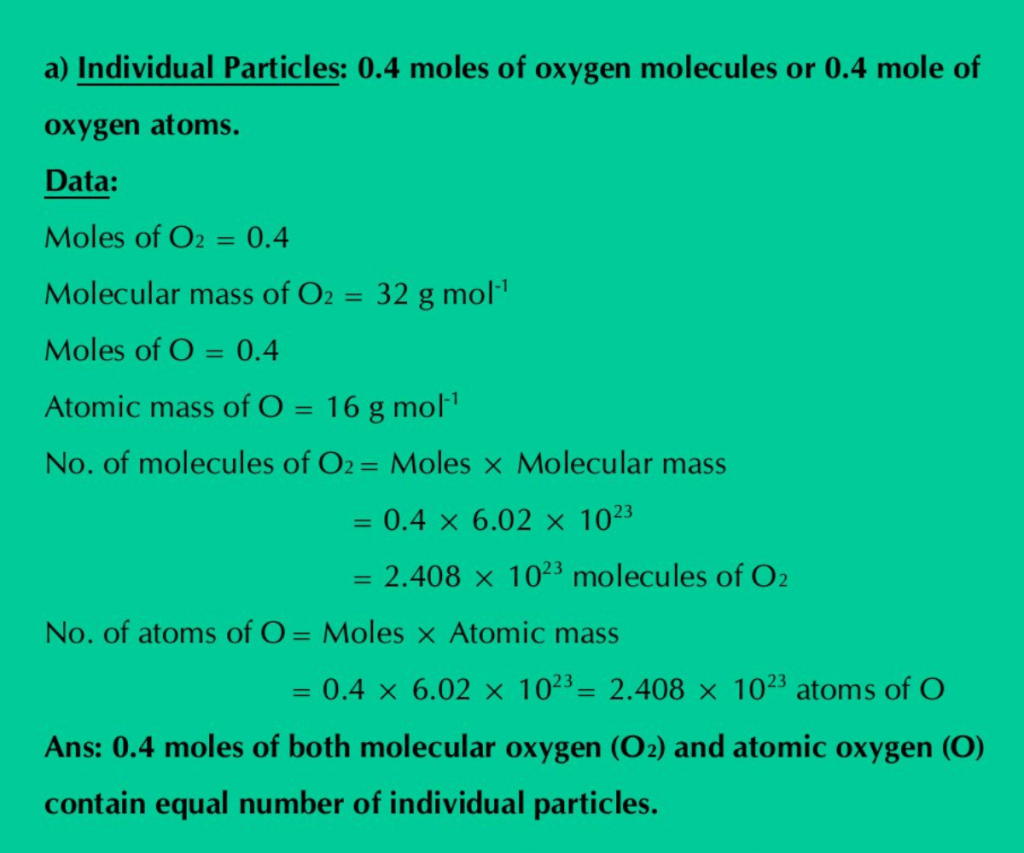
a) Individual particles: 0.4 moles of oxygen molecules or 0.4 mole of oxygen atoms.
Solution:
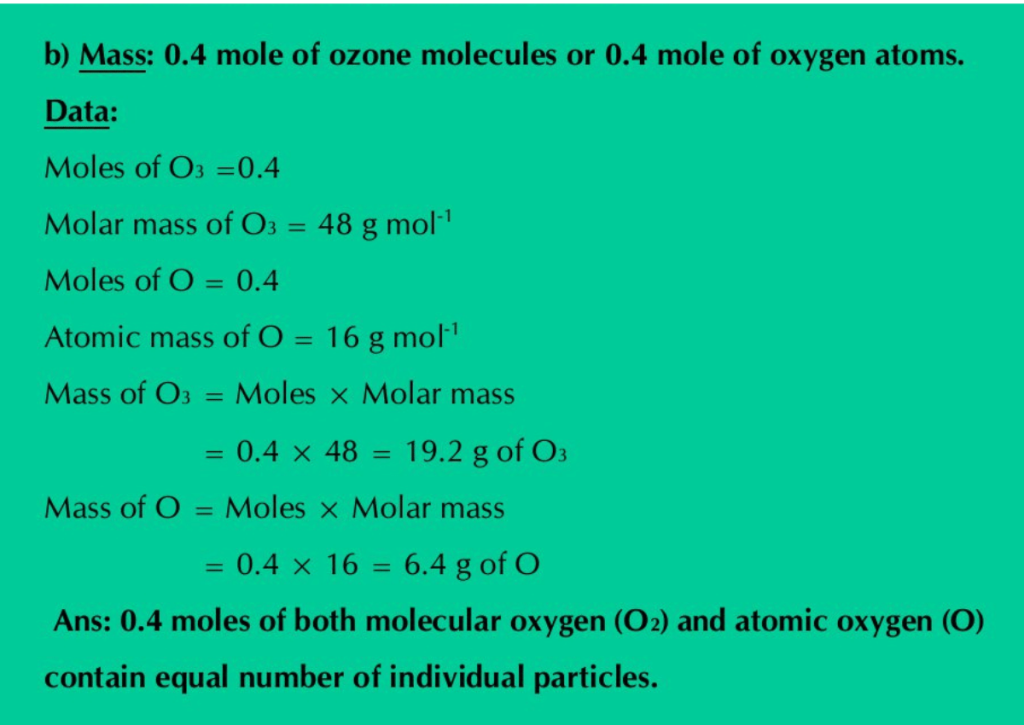
b) Mass: 0.4 mole of ozone molecules or 0.4 mole of oxygen atoms.
Solution:
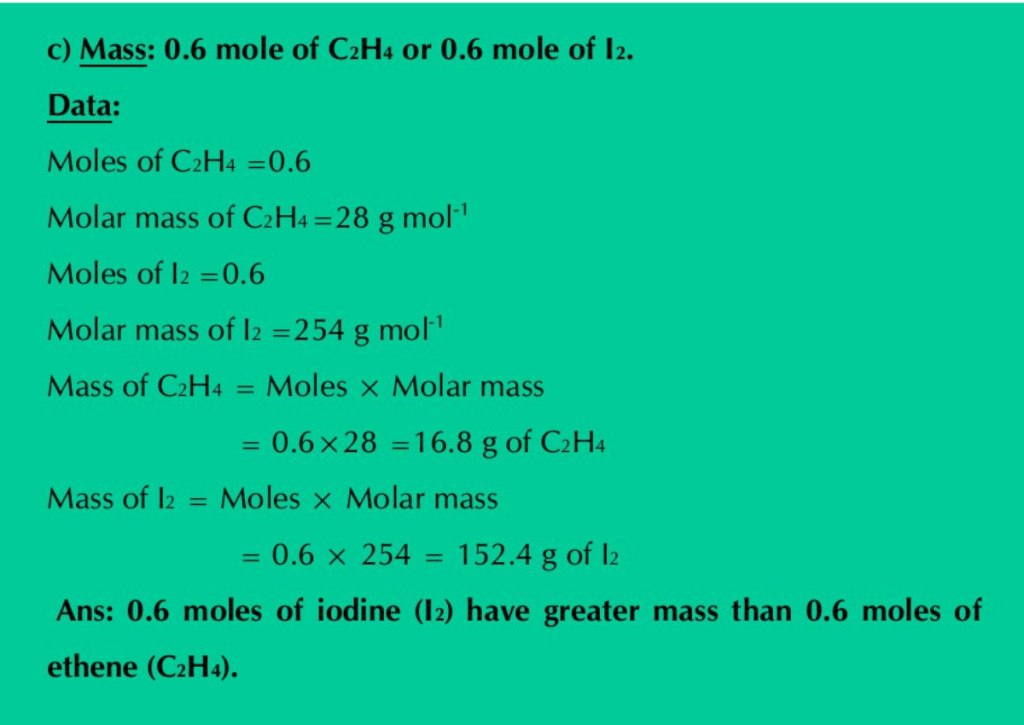
c) Mass: 0.6 mole of C2H4 or 0.6 mole of I2.
Solution:
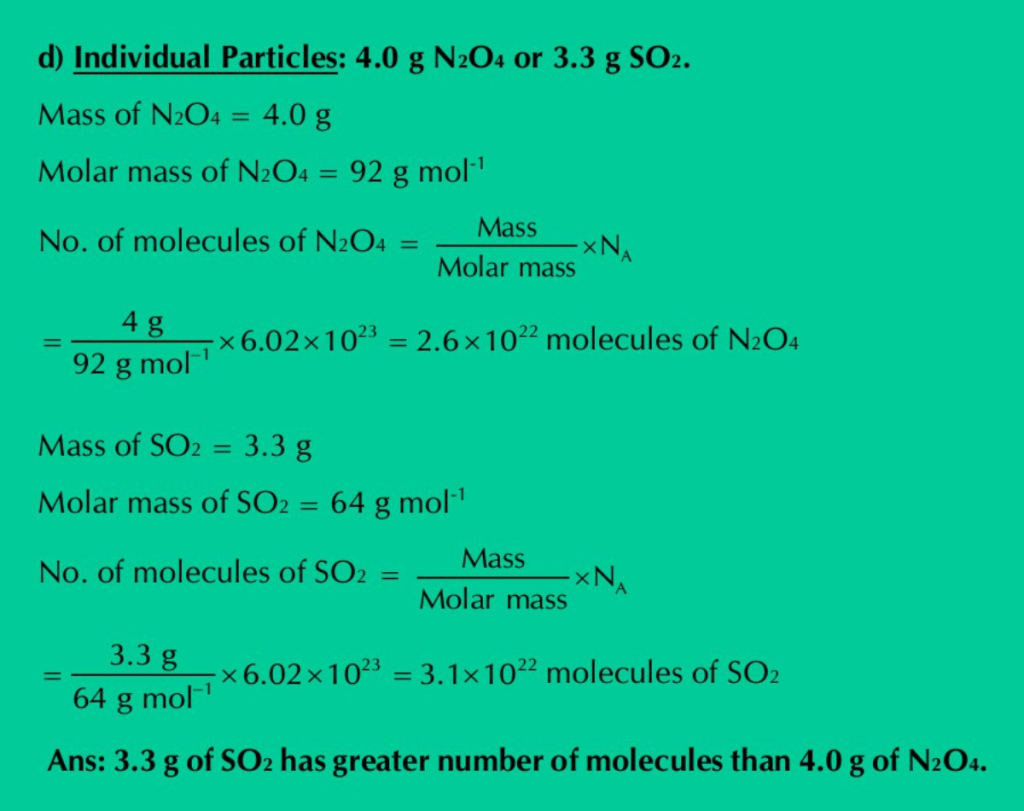
d) Individual particles: 4.0 g N2O4 or 3.3 g SO2.
Solution:
e) Total ions: 2.3 moles of NaClO3 or 2.0 moles of MgCl2.
Solution:

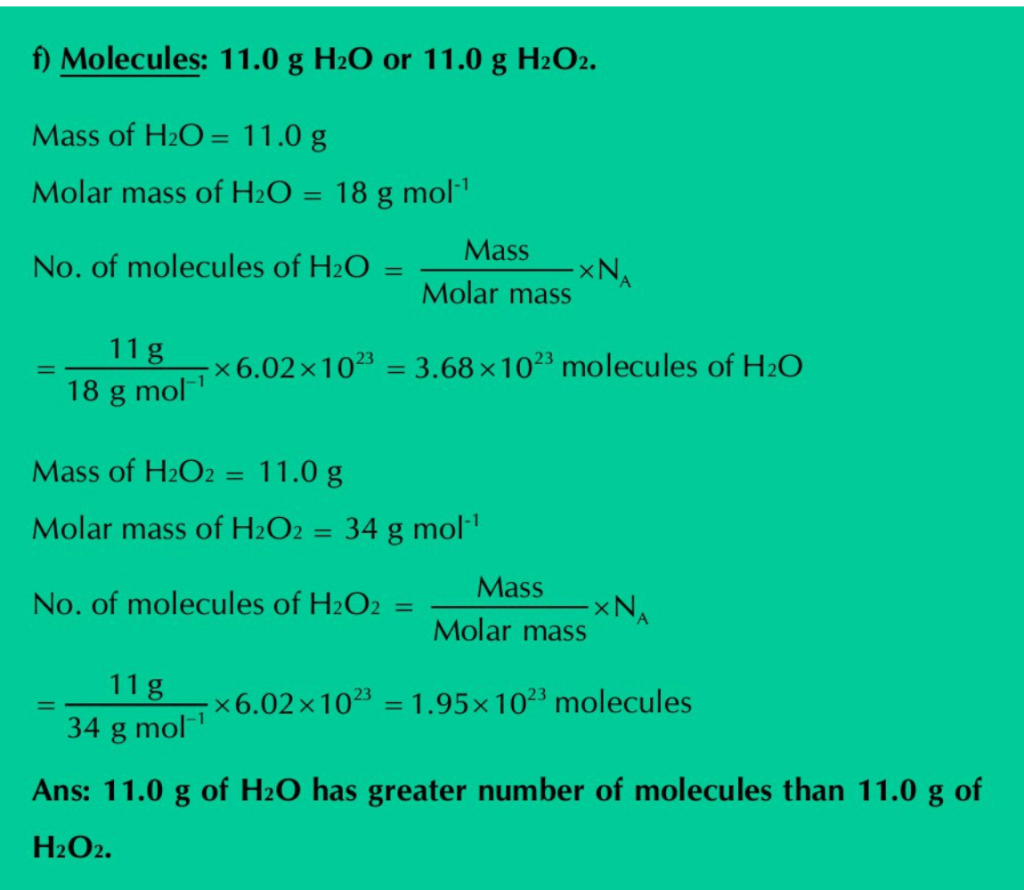
f) Molecules: 11.0 g H2O or 11.0 g H2O2.
Solution:
g) Na+ ion: 0.500 moles of NaBr or 0.0145 kg of NaCl.
Solution:

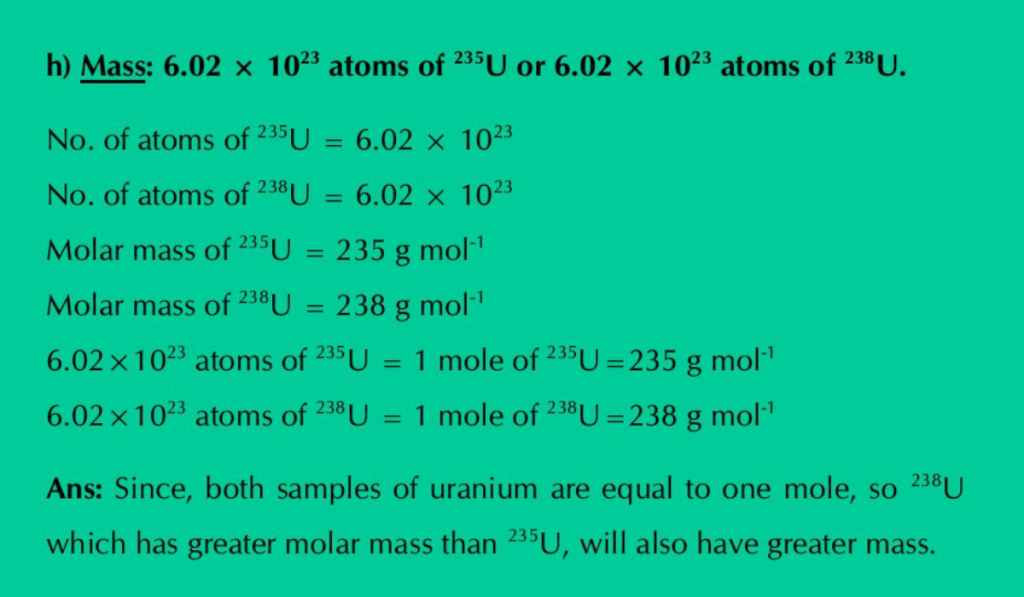
h) Mass: 6.02×1023 atoms of 235U or 6.02×1023 atoms of 235U.
Solution:
Q.14: a) Calculate the percentage of nitrogen in the four important fertilizers i.e. (i) NH3 (ii) NH2CONH2 (iii) (NH4)2SO4 (iv) NH4NO3
Solution:


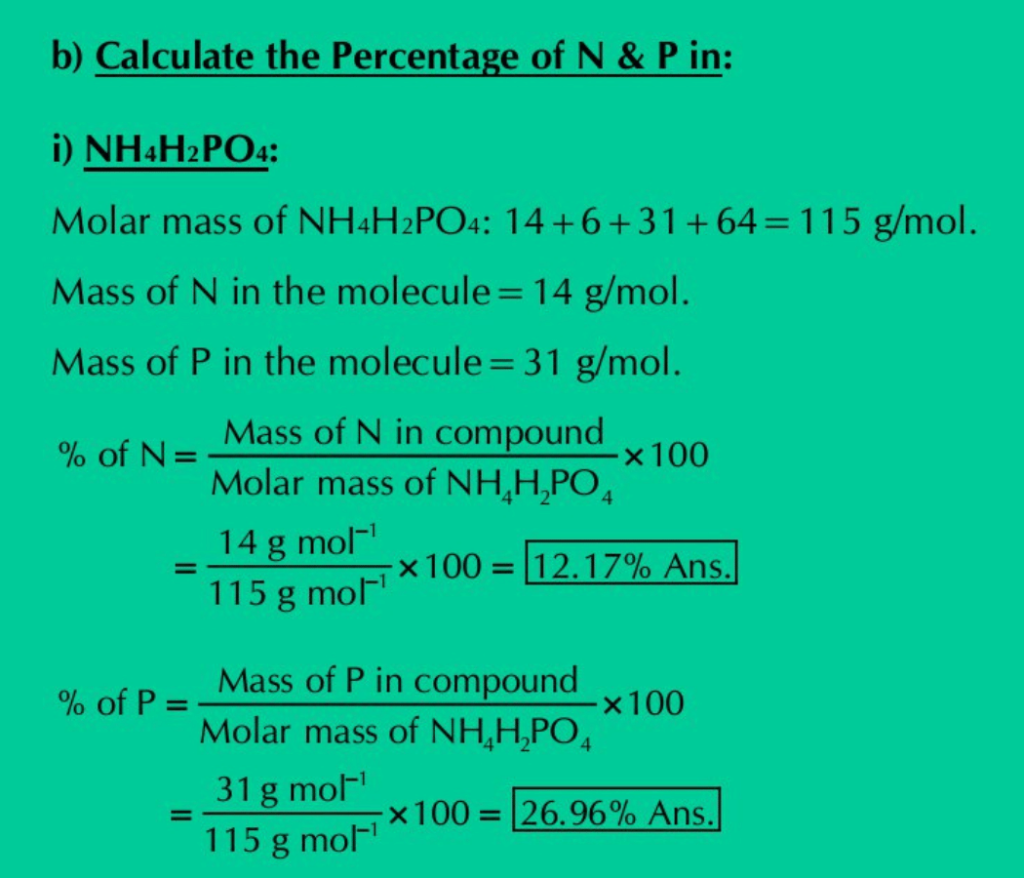
b) Calculate the percentage of nitrogen and phosphorus in each of the following:
(i) NH4H2PO4 (ii) (NH4)2HPO4 (iii) (NH4)3PO4
Solution:

Q.15: Glucose C6H12O6 is the most important nutrient in the cell for generating chemical potential energy. Calculate the mass % of each element in glucose and determine the number of C, H and O atoms in 10.5 g of the sample.
Solution:

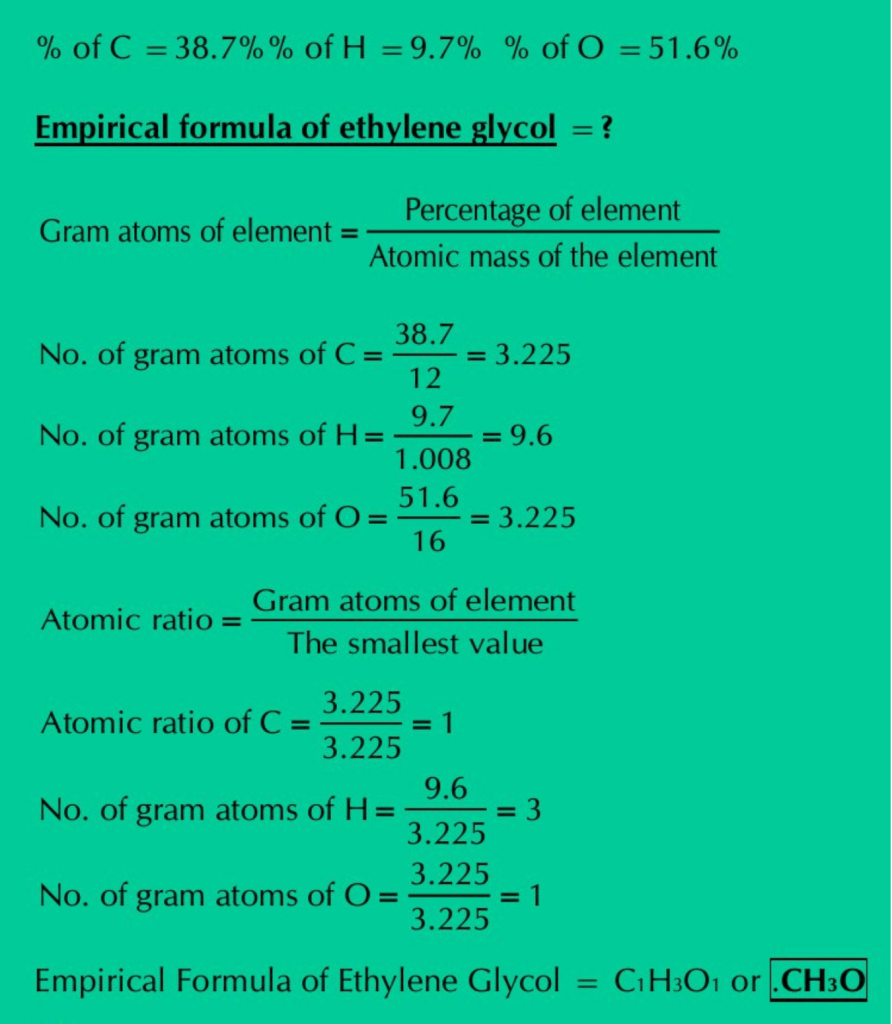
Q.16: Ethylene glycol is used as automobile antifreeze. It has 38.7% carbon, 9.7% hydrogen and 51.6% oxygen. Its molar mass is 62.1 g mol-1. Determine its empirical formula?
Solution:
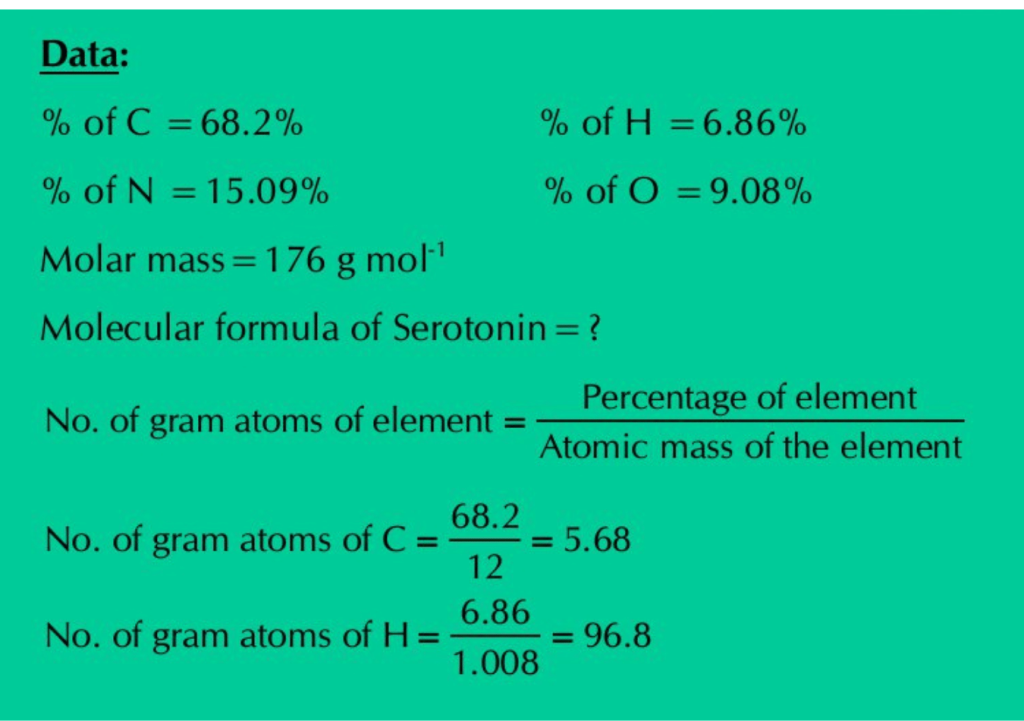
Q.17: Serotonin (Molar mass=176 g mol-1) is a compound that conducts nerve impulses in brain and muscles. It contains 68.2% C, 6.86% H, 15.09% N, and 9.08% O. What is its molecular formula?
Solution:

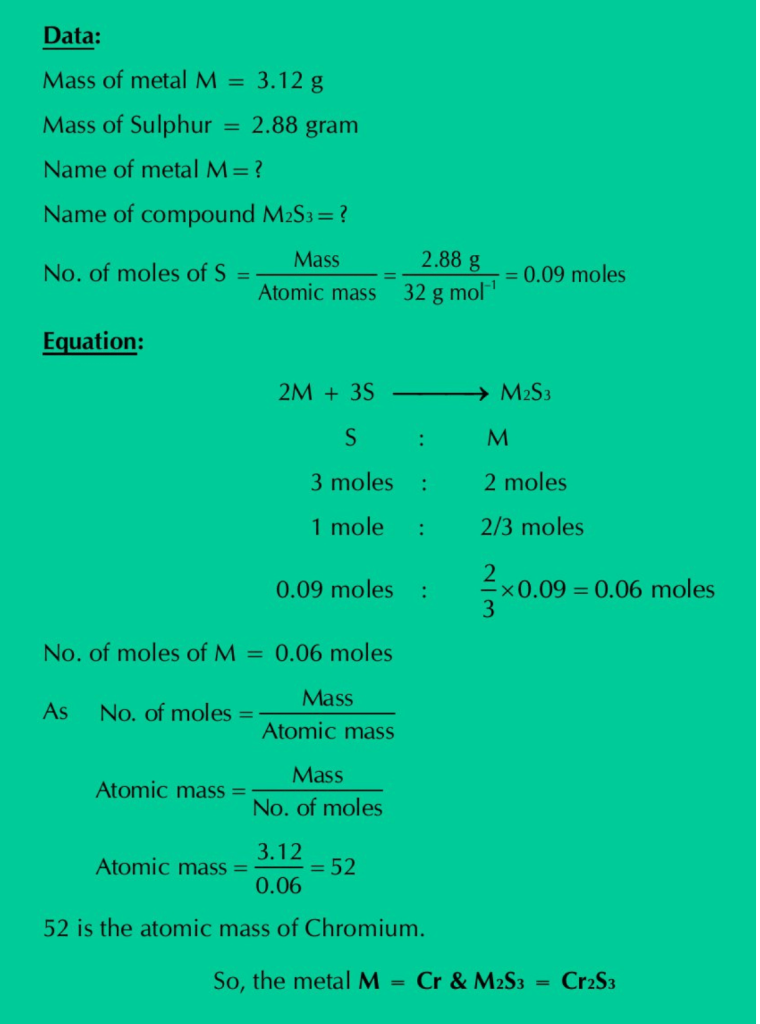
Q.18: An unknown metal M reacts with S to form a compound with a formula M2S3. If 3.12 g of M reacts with exactly 2.88 g of Sulphur, what are the names of metal M and the compounds M2S3?
Solution:
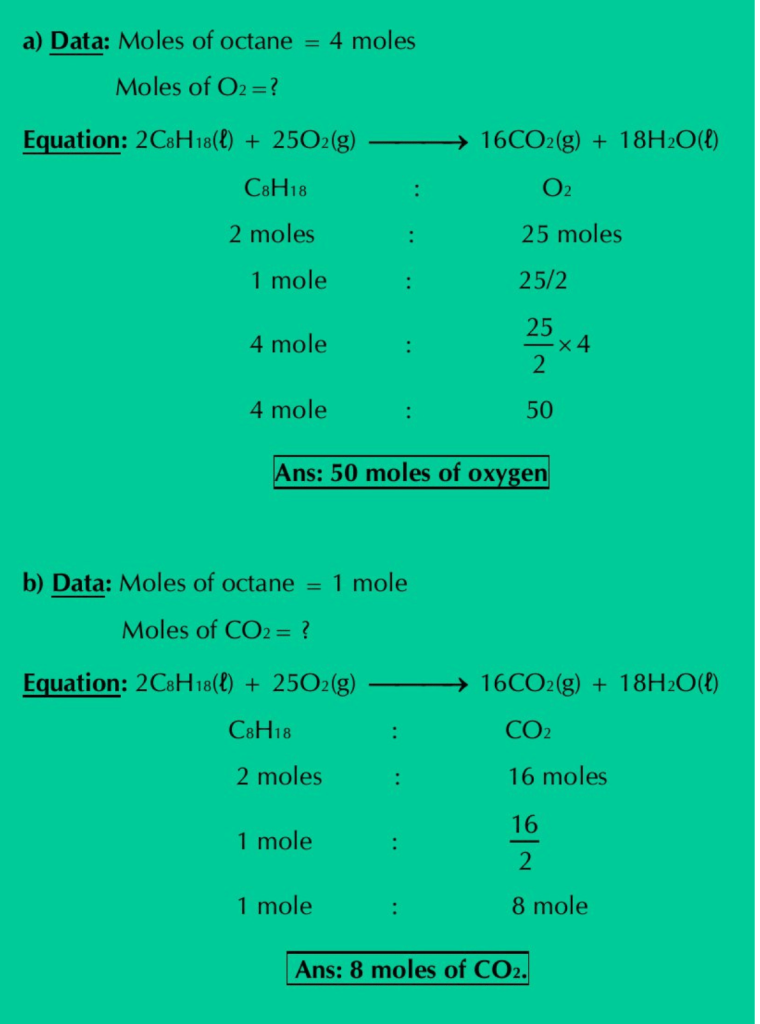
Q.19: The octane present in gasoline burns according to the following equation:
2C8H18(ℓ) + 25O2(g) ⟶ 16CO2(g) + 18H2O(ℓ)
a) How many moles of O2 are needed to react fully with 4 moles of octane?
b) How many moles of CO2 can be produced from one mole of octane?
c) How many moles of water are produced by the combustion of 6 moles of octane?
d) If this reaction is to be used to synthesize 8 moles of CO2, how many grams of oxygen are needed? How many grams of octane will be used?
Solution:

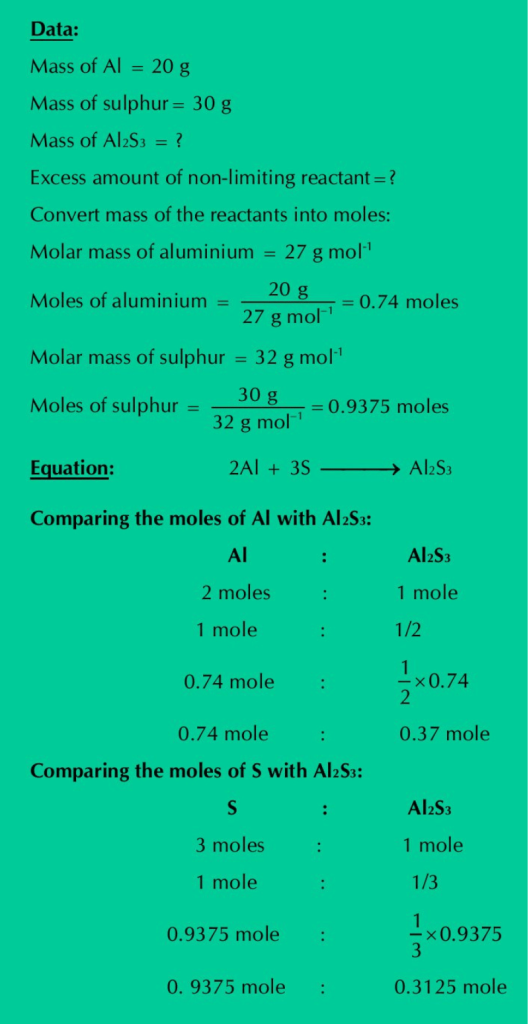
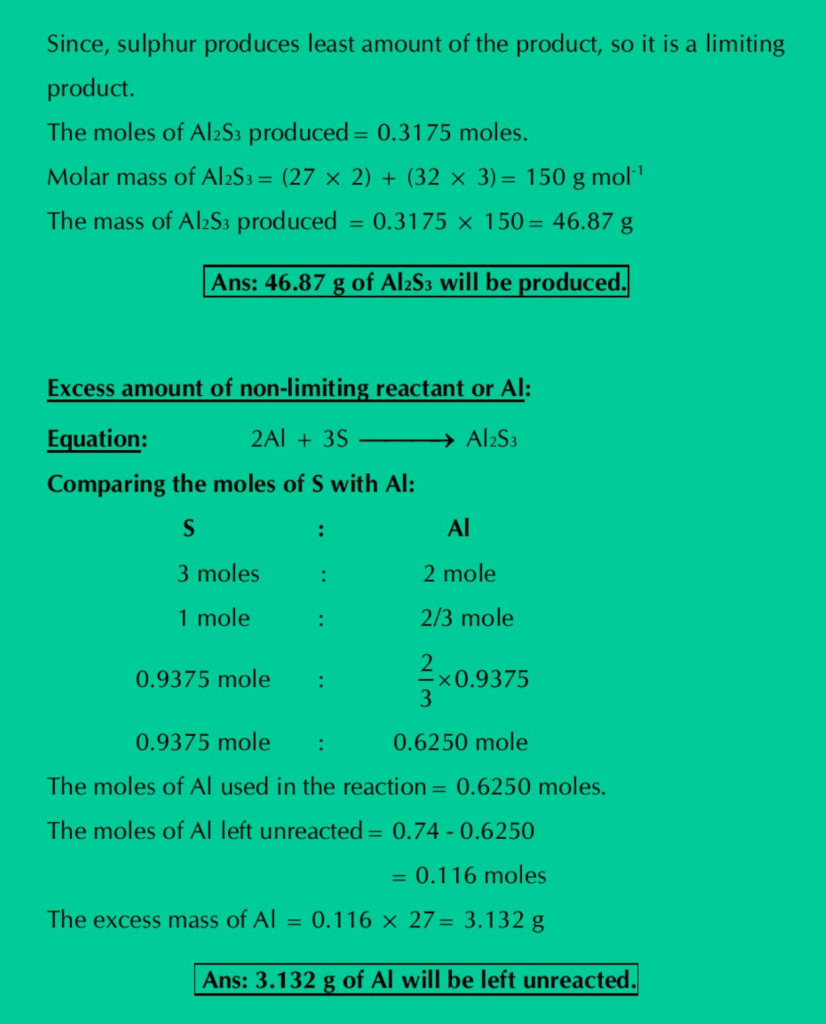
Q.20: Calculate the number of grams of Al2S3 which can be prepared by the reaction of 20 g of Al and 30 g of Sulphur. How much the non-limiting reactant is in excess?
Solution:
Q.21: A mixture of two liquids, hydrazine N2H4 and N2O are used in rockets. They produce N2 and water vapours. How many grams of N2 gas will be formed by reacting 100 g of N2H4 and 200 g of N2O4?
2N2H4 + N2O4 ⟶ 3N2 + 4H2O
Solution:


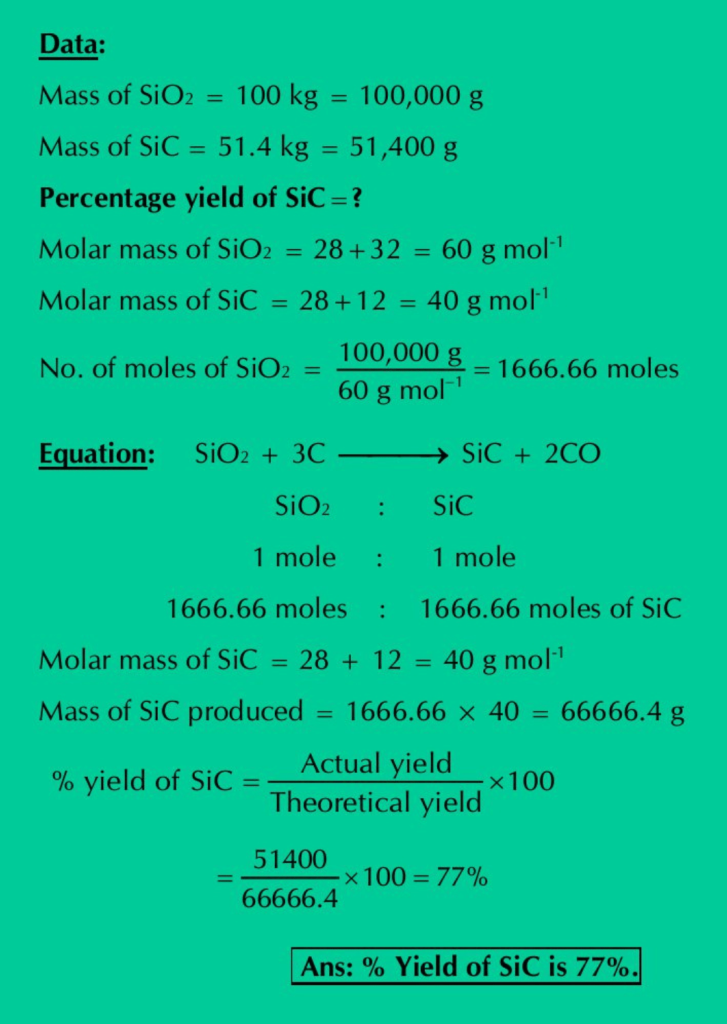
Q.22: Silicon carbide (SiC) is an important ceramic material. It is produced by allowing sand (SiO2) to react with carbon at high temperature.
SiO2 + 3C ⟶ SiC + 2CO
When 100 kg sand is reacted with excess of carbon, 51.4 kg of SiC is produced. What is the percentage yield of SiC?
Solution:
Q.23: a) What is stoichiometry? Give its assumptions? Mention two important laws, winch help to perform the stoichiometric calculations? b) What is a limiting reactant? How does it control the quantity of the product formed? Explain with three examples?
ANS:
a) STOICHIOMETRY: “Stoichiometry is the branch of chemistry which tells us the quantitative relationship between reactants and products in a balanced chemical equation.”
Basic Assumptions of Stoichiometry:
- All the reactants are converted into products.
- No side reaction occurs.
Laws of Stoichiometry: While doing stoichiometric calculations, the following two laws must be obeyed:
- Law of conservation of mass.
- Law of definite proportions.
b) LIMITING REACTANT: “The reactant which is consumed earlier in the chemical reaction and controls the amount of the product due to its smaller amount is called limiting reactant.”
Limiting reactant controls the amount of the product because the reaction proceeds as long as there is limiting reactant. When the limiting reactant is consumed, the reaction stops and no more product is formed. So, the amount of the product formed is determined by the limiting reactant.
Examples:
- If we have 30 “kababs” and 5 breads “having 58 slices”, then we can only prepare 29 “sandwiches”. One “kabab” will be extra (excess reactant) and “slices” will be the limiting reactant.
- Consider the reaction:
2H2(g) + O2(g) ⟶ 2H2O(ℓ)
If 2 moles of H2 are allowed to react with 2 moles of O2, only 2 moles of water will be formed. This is because 2 moles of H2 require only 1 mole of O2 to form 2 moles of water. 1 mole of O2 will be left unreacted. So, H2 is the limiting reactant because it is consumed earlier and controls the amount of the product. In the burning of wood in free supply of oxygen, wood is the limiting reactant because burning continues until there is wood. When the wood is consumed, the reaction stops.
Q.24: a) Define yield. How do we calculate the percentage yield of a chemical reaction? b) What are the factors which are mostly responsible for the low yield of the products in chemical reactions?
ANS: a) Yield: “The amount of the products obtained in a chemical reaction is called yield of that reaction.”
The efficiency of a chemical reaction is calculated by comparing the actual and theoretical yields, in the form of percentage yield.

The greater is the percentage yield, the greater is the efficiency, and vice versa.
b) In most reactions, actual yield remains less than the theoretical yield because of the following factors:
- A practically inexperienced worker has many shortcomings and cannot get the expected yield.
- The processes like filtration, separation by distillation, separation by a separating funnel, washing, drying and crystallization if not properly carried out, decrease the amount of the product.
- Some competing side reaction may waste some of the reactants
Q.25: Explain the following with reasons.
i) Law of conservation of mass has to be obeyed during stoichiometric calculations.
ANS: Law of conservation of mass has to be obeyed during stoichiometric calculations. This is because according to this law, reactants undergo a chemical change to give an equal amount of products. It means there is no creation or destruction of mass during the reaction. Thus, if we know the masses of the reactants, we can easily calculate the masses of the products and vice versa. Otherwise, if this law is not obeyed, the whole stoichiometric calculations will be wrong.
ii) Many chemical reactions taking place in our surrounding involve the limiting reactants.
ANS: Many chemical reactions taking place in our surrounding involve the limiting reactants. For example, burning of wood, rusting of iron, etc. In these reactions, one of the reactants is in excess. The other reactant which is present in less amount is the liming reactant. In burning of wood, air is excess reactant, while wood is the limiting reactant. In rusting of iron, air and moisture are excess reactants, while iron is the limiting reactant.
iii) No individual neon atom in the sample of the element has a mass of 20.18 amu.
ANS: Neon has three isotopes Ne–20, Ne–21 and Ne–22, present in the percentage of 90.92%, 0.26% and 8.82% respectively. When we calculate the average of all these isotopic masses, it comes out to be 20.18 amu.
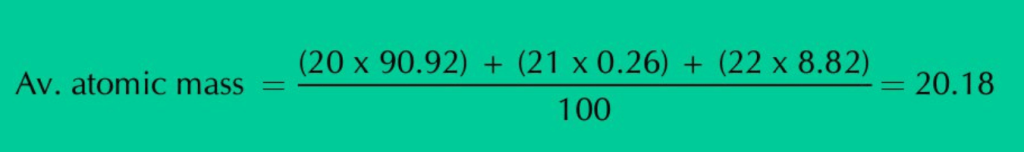
So, 20.18 amu is the average atomic mass of Neon, not the mass of any individual Neon atom.
iv) One mole of H2SO4 should completely react with two moles of NaOH. How does Avogadro’s number help to explain it.
ANS: One mole of H2SO4 should completely react with two moles of NaOH. This is because one mole of H2SO4 produces two moles of H+ ions.
H2SO4 ⇌ 2H+ + SO42-
While one mole of NaOH produces only mole of OH– ion.
NaOH ⇌ Na+ + OH–
Since, two moles H+ ions require two moles OH– ions for complete neutralization, so one mole of H2SO4 requires two moles of NaOH to react completely.
H2SO4 + 2NaOH ⟶ Na2SO4 + 2H2O
v) One mole of H2O has two moles of bonds, three moles of atoms, ten moles of electrons and twenty-eight moles of the total fundamental particles present in it.
ANS: One mole of H2O has two moles of bonds, three moles of atoms, ten moles of electrons and twenty-eight moles of the total fundamental particles present in it. This can be explained as:
Since, one molecule of H2O contains two O—H bonds, so one mole of H2O has two moles of covalent bonds i.e., 2 ✕ 6.02 ✕ 1023 bonds.
Since, one molecule of H2O contains three (2H+1O) atoms, so one mole of H2O has three moles of atoms i.e., 3 ✕ 6.02 ✕ 1023 atoms.
Since, one molecule of H2O contains 2+8=10 electrons, so one mole of H2O has ten moles of electrons i.e., 3 ✕ 6.02 ✕ 1023 electrons.
Since, one molecule of H2O contains 10 electrons (2+8=10), 10 protons (2+8=10) and 8 neutrons (H atoms have no neutrons), so one mole of H2O has twenty-eight moles of all three fundamental particles. i.e., 28 ✕ 6.02 ✕ 1023 fundamental particles.
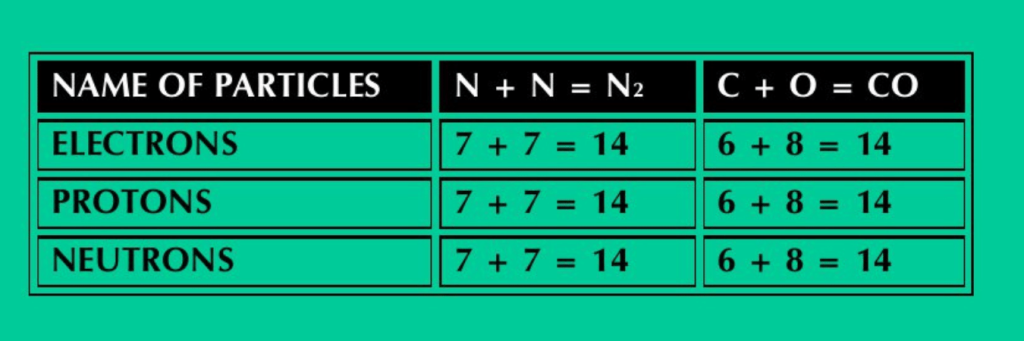
vi) N2 and CO have the same number of electrons, protons and neutrons.
ANS: N2 and CO have the same number of electrons, protons and neutrons. This can be verified by adding the number of electrons, protons and neutron in N, C & O atoms in these molecules.