ALIPHATIC HYDROCARBONS
FILL IN THE BLANKS
Q.01: Fill in the blanks:
(i) Ozone reacts with ethene to form ______. (ethylene ozonide)
(ii) Lindlar’s catalyst is used for _______ of alkynes. (partial hydrogenation)
(iii) Divinyl acetylene is a _________ acetylene. (polymer of)
(iv) Vicinal dihalides have two halogens on ______ carbon atoms. (adjacent)
(v) Ethyne is acidic in character because of _______ hybridization. (sp)
(vi) Halohydrins are formed due to addition of _______ in ethene. (hypohalous acid)
(vii) Ethylene glycol is produced when ______ reacts with cold alkaline KMnO4 solution. (ethene)
(viii) Mustard gas is a high boiling ________. (liquid)
(ix) Ethyne has ______ like odour.
(x) Ethyne is obtained by the reaction of ______ with calcium carbide. (garlic)
TRUE/FALSE
Q.02: Indicate True or False:
(i) Addition of HX to unsymmetrical alkanes takes place according to Markovnikov’s rule. (FALSE)
CORRECT: Addition of HX to unsymmetrical alkenes takes place according to Markovnikov’s rule.
(ii) Methane reacts with bromine water and its colour is discharged. (FALSE)
CORRECT: Ethene reacts with bromine water and its colour is discharged.
(iii) Mustard gas is a blistering agent. (TRUE)
(iv) Methane is also called marsh gas. (TRUE)
(v) Ethyne is a saturated compound. (FALSE)
CORRECT: Ethyne is an unsaturated compound.
(vi) Baeyer’s reagent is used to locate a double bond in an alkene. (FALSE)
CORRECT: Ozonolysis reaction is used to locate a double bond in an alkene.
(vii) Alkanes usually undergo substitution reactions. (TRUE)
(viii) Benzene is a polymer of ethene. (FALSE)
CORRECT: Benzene is a not a polymer of ethene.
(ix) Acrylonitrile can be obtained from ethyne. (TRUE)
(x) Ethyne is more reactive towards electrophilic reagents than ethene. (FALSE)
CORRECT: Ethyne is less reactive towards electrophilic reagents than ethene.
MCQs
Q.03: Multiple choice questions.
(i) Preparation of vegetable ghee involves:
(a) Halogenation
(b) Hydrogenation
(c) Hydroxylation
(d) Dehydrogenation
ANSWER: (b) Hydrogenation
EXPLANATION: In vegetable oil, unsaturated fatty acids are saturated by hydrogenation. So, it is converted into vegetable ghee.
(ii) Formula of chloroform is:
(a) CH3Cl
(b) CCl4
(c) CH2Cl2
(d) CHCl3
ANSWER: (d) CHCl3
EXPLANATION: Trichloromethane is commonly called chloroform. It is formed during the chlorination of methane in the presence of sunlight.
(iii) The presence of double bond in a compound is the sign of:
(a) Saturation
(b) Unsaturation
(c) Substitution
(d) None
ANSWER: (b) Unsaturation
EXPLANATION: The hydrocarbons containing double or triple bonds can add hydrogen atoms to their double or triple bonds to satisfy fully their tetravalency by making single bonds with four different atoms. So, they are called unsaturated compounds.
(iii) Vinyl acetylene combines with HCl to form:
(a) Poly acetylene
(b) Benzene
(c) Chloroprene
(d) Divinyl acetylene
ANSWER: (c) Chloroprene
EXPLANATION: CH2=CH—C≡CH + HCl ⟶ CH2=CH—C(Cl)=CH2 (Chloroprene)
(iv) The addition of unsymmetrical reagent to an unsymmetrical alkene is in accordance with the rule:
(a) Hund’s rule
(b) Markownikov’s rule
(c) Pauli’s Exclusion principle
(d) Aufbau principle
ANSWER: (b) Markownikov’s rule
EXPLANATION: According to Markownikov’s rule, in the addition of unsymmetrical reagent to an unsymmetrical alkene, the negative part of the reagent goes to that carbon, constituting the double bond, which has least number of hydrogen atoms.
(v) Synthetic rubber is made by polymerization of:
(a) Chloroform
(b) Acetylene
(c) Divinyl acetylene
(d) Chloroprene
ANSWER: (d) Chloroprene
EXPLANATION: When HCl is added to vinyl acetylene, chloroprene is obtained which readily polymerizes to neoprene, uses as synthetic rubber. Chloroprene ⟶ Neoprene (Synthetic rubber)
(vi) β,β-dichloroethyl sulphide is commonly known as:
(a) Mustard gas
(b) Laughing gas
(c) Phosgene gas
(d) Bio gas
ANSWER: (a) Mustard gas
EXPLANATION: β,β-Dichloroethyl sulphide or 2,2-Dichloroethyl sulphide is known as mustard gas because of its mustard like odour. It is actually a high boiling liquid which was used as a war gas in World War-I. It is a powerful vesicant i.e., causes blisters.
(vii) When methane reacts with Cl2 in the presence of diffused light the products obtained are:
(a) Chloroform only
(b) Carbon tetrachloride only
(c) Chloromethane and dichloromethane
(d) Mixture of a, b, c
ANSWER: (d) Mixture of a, b, c
EXPLANATION: The reaction between Cl2 and methane in the presence of sunlight is a free radical chain reaction which gives a variety of products such as CH3Cl, CH2Cl2, CHCl3 & CCl4.
(viii) Which one of the following gases is used for artificial ripening of fruits?
(a) Ethene
(b) Ethyne
(c) Methane
(d) Propane
ANSWER: (a) Ethene
EXPLANATION: Ethene is used for both natural and artificial ripening of fruit. In some countries, ethyne is also used as an alternative due to its cost effectiveness, but it has serious health repercussions.
ALKANES
NOMENCLATURE
Q.08:(a) Explain why alkanes are less reactive than alkenes? What is the effect of branching on the melting point of alkanes?
ANSWER:
The melting and boiling points of branched chain alkanes are lower than that of straight chain alkanes. This is because branched chain alkanes have low surface area. Their electrons are less exposed to be polarized by other molecules. So, they have weak intermolecular forces, and have lower melting and boiling points. For example, the melting and boiling points of n-butane are -138oC and -0.5oC, while that of iso-butane are -159oC and -11.7oC. The less reactivity of alkanes as compared to alkenes is due to two reasons. First, the bonds in alkanes are nearly non-polar due to little E.N. difference between C and H. So, the ionic reagents like acids, alkalies and oxidizing agents find no place on alkanes to attack. Second, all the bonds in alkanes are sigma which are very strong and inert. The sigma electrons are held tightly between the two nuclei. So, no electrophile can attack them.
(b) Three different alkanes yield 2-methylbutane when they are hydrogenated in the presence of a metal catalyst. Give their structures and write equations for the reactions involved.
ANSWER:
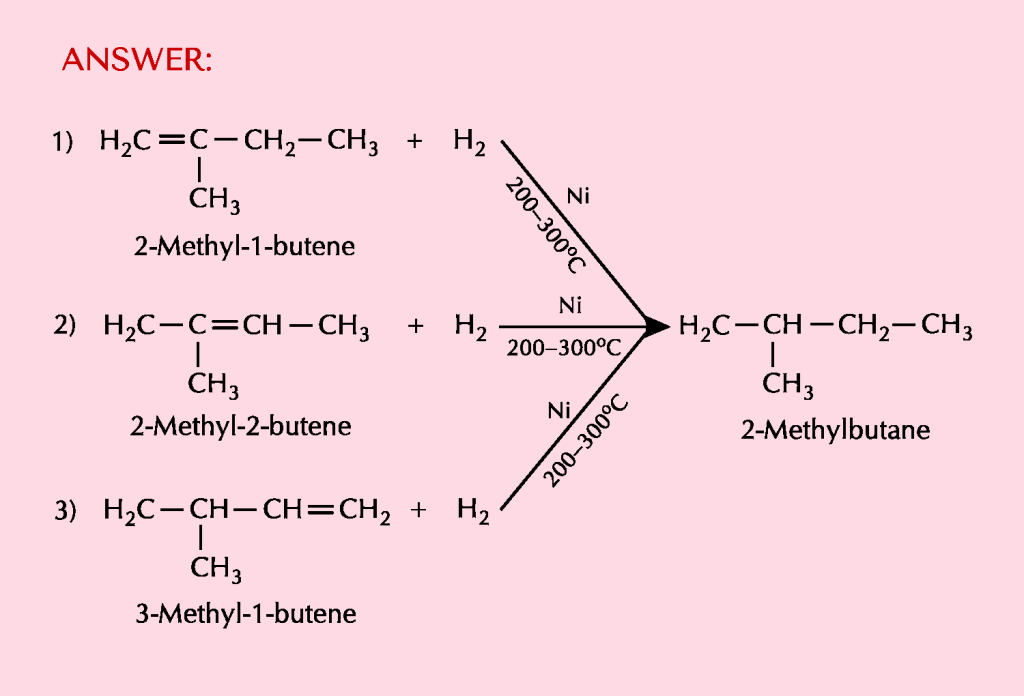
ALKENES
ALKYNES

