CHEMICAL BONDING
Q.01: Select the most suitable answer for the given one.
1) An ionic compound A+B– is most likely to be formed when:
(a) The ionization energy of A is high and electron affinity of B is low.
(b) The ionization energy of A is low and electron affinity of B is high.
(c) Both the ionization energy of A and electron affinity of B are high.
(d) Both the ionization energy of A and electron affinity of B are low.
Ans: (b) The ionization energy of A is low and electron affinity of B is high.
EXPLANATION: The atom with low ionization energy will make positive ion very easily. Similarly, an other atom with high electron affinity will make negative ion easily because it has great affinity or attraction for the eletcrons. Thus, if A has low value of ionization energy and B has greater value of electron affinity, most likely, they make an ionic bond as A+B–.
(2) The number of bonds in nitrogen molecule is:
(a) One sigma and one pi
(b) One sigma and two pi
(c) Three sigma only
(d) Two sigma and one pi
Ans: (b) one sigma and two pi
EXPLANATION: In N2 molecule, there are total three covalent bonds between two nitrogen atoms (N≡N). When two atoms make multiple bonds, the first bond will always be sigma which is the result of linear overlap between valence orbitals, and the second and third bonds will be pi because after the formation of sigma bond, the remaining orbitals can overlap only parallel fashion.
(3) Which of the following statements is not correct regarding bonding molecular orbital?
(a) Bonding molecular orbitals possess less energy than atomic orbital from which they are formed.
(b) Bonding molecular orbitals have low electron density between the two nuclei.
(c) Every electron in bonding molecular orbitals contributes to the attraction between the atoms.
(d) Bonding molecular orbitals are formed when the electron waves undergo constructive interference.
Ans: (b) Bonding molecular orbitals have low electron density between the two nuclei.
EXPLANATION: Bonding molecular orbitals are formed around the line joining the two nuclei. So, the electron density of bonding electrons will be maximum in this region.
(4) Which of the following molecules has zero dipole moment?
(a) NH3
(b) CHCl3
(c) H2O
(d) BF3
Ans: (d) BF3
EXPLANATION: BF3 has triangular planar structure. The three bonds arranged at 120o to one another. The dipole moments of three bonds, being opposite in direction, cancel each other. So, the net dipole moment is equal to 0D.
(5) Which of the hydrogen halides has the highest percentage of ionic character?
(a) HCl
(b) HBr
(c) HF
(d) HI
Ans: (c) HF
EXPLANATION: HF has the highest percentage of ionic charcater because the electronegative difference between H and F atoms is maximum. The greater is the E.N. difference between two bonded atoms, the greater is the percentage of ionic character of the bond and vice versa.
(6) Which of the following species has unpaired electrons in antibonding molecular orbital?
(a) O22+
(b) N22-
(c) B2
(d) F2
Ans: (b) N22-
EXPLANATION: In N22-, there are total 16 electrons. Filling these elertcrons in molecular orbitals in increasing order of energy, the final two electrons remain unpaired in π★2py and π★2pz. Thus, N22- has two unpaired eletcrons.
FILL IN THE BLANKS
Q.02: Fill in the blanks:
(1) The tendency of atoms to attain maximum of ________ electrons in the valence shell, is called completion of octet. (Eight)
(2) The geometrical shape of SiCl4 and PCl3 can be explained on the basis of _______ and _______ hybridizations. (sp3 & sp2)
(3) The VSEPR theory stands for___________. (Valence Shell Electron Pair Repulsion Theory)
For N2 molecule, the energy of s(2px) orbital is ____________ than p(2py) orbital. (Higher)
(4) The paramagnetic property of O2 is well explained on the basis of MO theory in terms of the presence of ___________ electrons in two MO orbitals. (Two unpaired)
(5) The value of dipole moment for CS2 is _______ while for SO2 is _______. (0 D / 1.61 D)
(6) The bond order of N2 is ________, while that of Ne2 is ________. (three / zero)
Q.03: Classify the statements as true or false. Explain with reasons.
(1) The core of an atom is the atom minus its valence shell. (FALSE)
EXPLANATION: The core of atom means nucleus, the inner part of atom.
(2) The molecules of nitrogen (N≡N) and acetylene (HC≡CH) are not isoelectronic. (FALSE)
EXPLANATION: The molecules of N2 and C2H2 are isoelectronic as both have 14 electrons each.
(3) There are four coordinate covalent bonds in NH4+ ion. (FALSE)
EXPLANATION: There is only one co-ordinate covalent bond in NH4+ ion, while the remaining three are normal covalent bonds. However, the distinction between covalent and co-ordinate covalent bonds vanishes because all bonds are formed between similar atoms. So, every bond is 75% covalent and 25% co-ordinate.
(4) A s-bond is stronger than a p-bond and the electrons of s-bond are more diffused than p-bond. (FALSE)
EXPLANATION: Sigma bond is stronger than pi bond but sigma electrons are less diffused and more concentrated as compared to pi electrons.
(5) The bond energy of heteroatomic diatomic molecules increases with the decrease in the electro negativities of the bonded atoms. (FALSE)
EXPLANATION: The bond energies of heteroatomic diatomic molecules decrease as the E.N. difference between the bonded atoms decreases.
(6) With increase in bond order, bond length decreases and bond strength increases. (TRUE)
(7) The first ionization energies of the elements rise steadily with the increasing atomic number from top to bottom in a group. (FALSE)
EXPLANATION: From top to bottom in a group, the first ionization energies decrease with the increase in atomic number. This is due to the increase in the number of shells and atomic size down the group.
(8) A double bond is stronger than a single bond and a triple bond is weaker than a double bond. (FALSE)
EXPLANATION: Tripple bond is even stronger than double bond because it has greater extent of overlapping or orbitals. So, the bond length decreases and the bond strength increases.
(9) The bonds formed between the elements having electronegativity difference more than 1.7 are said to be covalent in nature. (FALSE)
EXPLANATION: The bonds formed between two atoms having E.N. difference greater than 1.7 are said to be ionic rather than covalent in nature.
(10) The repulsive force between two bonding pairs is less than that between two lone pairs. (TRUE)
(11) The number of covalent bonds an atom can form is related to the number of unpaired electrons it has. (TRUE)
(12) The rules which govern the filling of electrons into the atomic orbitals also govern filling of electrons into the molecular orbitals. (TRUE)
Q.18: Explain the following with reasons:
(i) Bond distance is the compromise distance between two atoms.
ANSWER: Bond distance is the compromise distance between two atoms. This is because when two atoms approach each other to make a bond, their P.E. energy decreases. On reaching a certain close distance, their energy becomes minimum and the attraction between them becomes maximum. At that distance, both the atoms compromise to make bonds because here the system is the most stable. That’s why, this distance between two bonded atoms which is about 75.4 pm in case of H-atoms, is termed as compromise bond distance or simply the bond distance.
(ii) The distinction between a coordinate covalent bond and a covalent bond vanishes after bond formation in NH4+, H3O+ and CH3NH3+.
ANSWER: In H3O+, there are three bonds; two covalent and one co-ordinate. All these bonds are between similar atoms (O & H), so the distinction between the bonds vanishes. The co-ordinate character is equally distributed between all these bonds. Thus, each bond is 33.3% co-ordinate and 66.6% covalent. In , there are four bonds; three covalent and one co-ordinate. All these bonds are between similar atoms (N and H), so the distinction between the bonds vanishes. The co-ordinate character is equally distributed between all these bonds. Thus, each bond is 25% co-ordinate and 75% covalent.
(iii) The bond angles of H2O and NH3 are not 109.5° like that of CH4. Although, O– and N– atoms are sp3 hybridized.
ANSWER: In CH4, NH3 and H2O, the C, N & O atoms are sp3 hybridized, but the bond angles in all of them are different. This is because in CH4, all sp3 hybrid orbitals have single electrons which form four single bonds with four H-atoms. None of the hybrid orbitals have a lone pair of electrons. The bond pairs exert equal repulsions, so they arrange tetrahedrally at an angle of 109.5o. In NH3, however, one of the four sp3 hybrid orbitals has a lone pair of electrons, which exerts greater repulsion, so reduces the angle between three bond pairs to 107.5°. In H2O, two of the sp3 hybrid orbitals have two lone pairs, which exert even greater repulsion on two bond pairs. The molecule becomes bent or angular with bond angle of 104.5o.

(iv) p-bonds are more diffused than s-bonds.
ANSWER: Pi bonds are more diffused than sigma bonds. This is because Pi bond is formed by the parallel overlap of two partially filled orbitals. Their electrons lie above and below the line joining the two nuclei. They are comparatively far from both the nuclei, so they are weakly attracted. This is the reason why pi electronic cloud is more diffused in space than sigma bond. On the other hand, sigma bond is formed by the linear overlap of two partially filled orbitals. Their electrons lie on the line joining the two nuclei. They are comparatively closer to both the nuclei, so they are strongly attracted. That’s why, sigma electronic cloud is not so diffused in space as pi bond.
(v) The abnormality of bond length and bond strength in HI is less prominent than that of HCl.
ANSWER: The abnormality of bond length and bond strength is less prominent in HI than in HCl. The reason is that due to greater E.N. difference, H–Cl bond is more polar than H–Br & H–I bonds. Since, polarity produces ionic character which gives additional strength to the bond. So, HCl shows greater decrease in bond length and greater increase in bond energy as compared to HBr & HI.
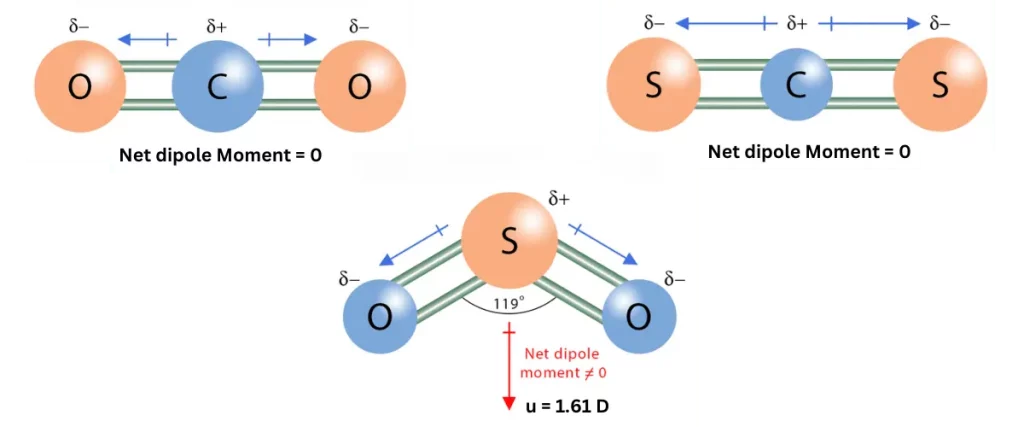
(vi) The dipole moments of CO2, and CS2 are zero, but that of SO2 is 1.61 D.
ANSWER: The dipole moment of CO2 and CS2 is zero because both CO2 and CS2 are linear molecules, having two double bonds between C and O/S. There is no lone pair of electrons on carbon. The dipole moments of two double bonds, being equal and opposite in direction, cancel each other. So, the net dipole moment becomes zero.
The dipole moment of SO2 is 1.61 D because it has bent or angular structure. This is due to the presence of a lone pair of electrons on sulphur, which repels the two bond pairs to arrange into a bent structure. The dipole moments do not cancel each other, so the molecule becomes polar with dipole moment 1.61 D.
(vii) The melting points, boiling points, heat of vaporizations and heat of sublimations of electrovalent compounds are higher as compared with those of covalent compounds.
ANSWER: The melting points, boiling points, heat of vaporizations and heat of sublimations of electrovalent or ionic compounds are higher because they have strong electrostatic forces of attraction between cations and anions. While, covalent compounds are in the form of molecules which are usually y held together through weak intermolecular forces such as hydrogen bonding, dipole-dipole interactions, London dispersion forces etc. Therefore, they are easy to melt and boil as well as to vaporize and sublimate.