FUNDAMENTAL PRINCIPLES OF ORGANIC CHEMISTRY
FILL IN THE BLANKS
Q.01: Fill in the blanks:
(i) Organic compounds having same molecular formula but different ______ are called isomers. (structure)
(ii) The state of hybridization of carbon atom in ______ is sp2. (ethene)
(iii) Alkenes show ______ due to restricted rotation around a carbon-carbon double bond. (geometric isomerism)
(iv) Heating an organic compound in the absence of oxygen and in the presence of ________ as a catalyst is called cracking. (SiO2+Al2O3)
(v) A group of atoms which confers characteristic properties to an organic compound is called _________. (function group)
(vi) 2-Butene is ________ of 1-Butene. (position isomer)
(vii) Carbonyl functional group is present in both _________ and ketones. (aldehydes)
(viii) A heterocyclic compound contains an atom other than ______ in its ring. (carbon)
(ix) The quality of gasoline can be checked by finding out its _______. (octane number)
(x) A carboxylic acid contains _______ as a functional group. (– COOH)
Q.02: Indicate True or False:
(i) There are three possible isomers for pentane. (TRUE)
(ii) Alkynes do not show the phenomenon of cis-trans isomerism. (TRUE)
(iii) Organic compounds can not be synthesized from inorganic compounds. (FALSE)
CORRECT: Organic compounds can be synthesized from inorganic compounds.
(iv) All close chain compounds are aromatic in nature. (FALSE)
CORRECT: All close chain compounds are not aromatic in nature.
(v) The functional group present in amides is called an amide group. (FALSE)
CORRECT: The functional group present in amides is called an amide group.
(vi) Government of Pakistan is trying to use coal for power generation. (TRUE)
(vii) Crude petroleum is subjected to fractional sublimation in order to separate it into different fractions. (FALSE)
CORRECT: Crude petroleum is subjected to fractional distillation in order to separate it into different fractions.
(viii) A bond between carbon and hydrogen serves as a functional group for alkanes. (FALSE)
CORRECT: Alkanes don’t have a functional group.
(ix) o-Nitrotoluene and p-nitrotoluene are the examples of functional group isomerism. (FALSE)
CORRECT: o-Nitrotoluene and p-Nitrotoluene are the examples of position isomerism.
(x) Almost all the chemical reactions taking place in our body are inorganic in nature. (FALSE)
CORRECT: Almost all the chemical reactions taking place in our body are organic in nature.
Q.03: Multiple choice questions.
(i) The state of hybridization of carbon atom in methane is:
(a) sp
(b) sp3
(c) sp2
(d) dsp2
ANSWER: (b) sp3
EXPLANATION: In alkanes, each carbon makes four single bonds with other atoms, which are arranged in a tetrahedral manner. Such a singly bonded carbon will always be sp3 hybridized because sp3 hybridization results in a set of four hybrid orbitals which are arranged in a tetrahedral shape.
(ii) In t-butyl alcohol, the tertiary carbon is bonded to:
(a) Two hydrogen atoms
(b) Three hydrogen atoms
(c) One hydrogen atom
(d) No hydrogen atom
ANSWER: (d) No hydrogen atom
EXPLANATION: A tertiary carbon is that which is bonded to four other carbon atoms. In tertiary butyl alcohol, the -OH group is attached to tertiary carbon which is attached to three other carbon atoms. In this way, tertiary carbon has no bond with hydrogen atom.
(iii) Which set of hybrid orbitals has planar triangular shape?
(a) sp3
(b) sp
(c) sp2
(d) dsp2
ANSWER: (c) sp2
EXPLANATION: In sp2 hybridization, the three hybrid orbitals are arranged in a triangular planar manner and the fourth unhybrid p-orbital lies perpendicular to all three hybrid orbitals.
(iv) The chemist who synthesized urea from ammonium cyanate was:
(a) Berzelius
(b) Kolbe
(c) Wohler
(d) Lavoisier
ANSWER: (c) Wohler
EXPLANATION: Friedrick Wohler, a German chemist, obtained urea (NH2)2CO, an organic compound in the urine of mammals, from ammonium cyanate, NH4CNO, a substance of known mineral origin.
(v) Linear shape is associated with which set of hybrid orbitals?
(a) sp
(b) sp2
(c) sp3
(d) sp3
ANSWER: (a) sp
EXPLANATION: In sp hybridization, the two hybrid orbitals are arranged in a linear shape and the remaining two unhybrid p-orbitals lie perpendicularly to two sp hybrid orbitals.
(vi) A double bond consists of:
(a) Two sigma bonds
(b) One sigma and one pi bond
(c) One sigma and two pi bonds
(d) Two pi bonds
ANSWER: (b) One sigma and one pi bond
EXPLANATION: In a double bond, the first bond formed results from head-to-head or linear overlap of partially filled orbitals, this is called sima bond. Then the unhybrid orbitals of bonded atoms make side-ways or parallel overlap to make a pi bond. In this way, a double bond contains one sigma and one pi bond.
(vii) Ethers show the phenomenon of:
(a) Position isomerism
(b) Functional group isomerism
(c) Metamerism
(d) Cis-trans isomerism
ANSWER: (c) Metamerism
EXPLANATION: The general formula of ether is R—O—R. R is alkyl group which may be same or different in different ethers. So, depending upon the nature of alkyl group around the O atom, ethers may have different metamers.
(viii) Select from the following the one which is alcohol:
(a) CH3-CH2-OH
(b) CH3-O-CH3
(c) CH3COOH
(d) CH3-CH2-Br
ANSWER: (a) CH3-CH2-OH
EXPLANATION: Alcohols have general formula R—OH. The presence of -OH or hydroxyl group attached carbon or alkyl group shows that the given compound is an alcohol.
Q.04: How organic compounds are classified? Give suitable example of each type.
ANSWER: Consult the textbook at page 123—125.
Q.05: What are homocyclic and heterocyclic compounds? Give one example of each.
ANSWER:
Homocyclic or Carbocyclic Compounds: The compounds in which the ring consists of only carbon atoms, homocyclic or carbocyclic compounds.
Examples: cyclobutene, benzene, naphthalene etc.
Heterocyclic Compounds: The compounds in which the ring consists of atoms of more than one kind are called heterocyclic compounds or heterocycles. In heterocyclic compounds generally one or more atoms of elements such as nitrogen (N), oxygen (O) or sulphur (S) are present. The atom other than carbon viz, N, 0, or S, present in the ring is called a hetero atom.
Examples: Pyridine, furan, pyrrole, thiophene etc.
Q.06: Write the structural formulas of the two possible isomers of C4H10.
ANSWER:
Isomers of Butane, C4H10
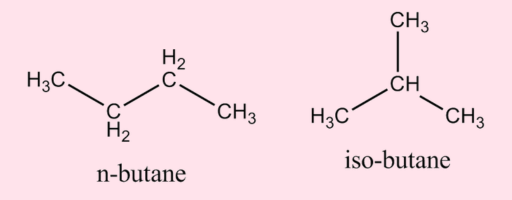
Q.07: Why is ethene an important industrial chemical?
ANSWER: Ethene an important industrial chemical because it is used:
(1) For the manufacture of polythene, a plastic material used for making toys, cables, bags, boxes, etc.
(2) For artificial ripening of the fruits.
(3) As a general anaesthetic.
(4) For preparing ‘Mustard gas’ a chemical used in World War I. The name comes from its mustard like odour. It is not a gas, but a high boiling liquid that is dispersed as a mist of tiny droplets. It is a powerful vesicant i.e., causes blisters.
(5) As a starting material for a large number of chemicals of industrial use such as glycols (antifreeze), ethyl halide, ethyl alcohol, etc.
Q.08: What is meant by a functional group? Name typical functional groups containing oxygen.
ANSWER:
Functional Group: “An atom or a group of atoms or a double bond or a triple bound whose presence imparts specific properties to organic compounds is called a functional group.” Functional groups are the functional parts of the organic molecules.
Examples of Oxygen Containing Functional Groups:
(1) Alcohols R—OH, Functional Group –OH.
(2) Ethers: R—OH, Functional Group –O–.
Q.09: What is an organic compound? Explain the importance of Wohler’s work in the development of organic chemistry.
ANSWER: Before Wohler’s work, organic compounds were obtained only from living organisms. Wohler prepared first organic compound urea, from an inorganic compound ammonium cyanate, in the laboratory. After that millions of organic compounds were made artificially. All this resulted from Wohler’s work in organic chemistry.
Q.10: Write a short note on cracking of hydrocarbons.
ANSWER: Consult textbook at page 122—123.
Q.11: Explain reforming of petroleum with the help of suitable example.
ANSWER: Consult textbook at page 123.
Q.12: Describe important sources of organic compounds.
ANSWER: Consult textbook at page 120—122.
Q.13: What is orbital hybridization? Explain sp3, sp2 and sp modes of hybridization of carbon.
ANSWER: Consult textbook at page 127—130.
Q.14: Explain the type of bonds and shapes of the following molecules using hybridization approach: CH3—CH3, CH2 =CH2, CH=CH, HCHO, CH3CI
ANSWER:
Q.15: Why there is no free rotation around a double bond and a free rotation around a single bond? Discuss cis-trans isomerism.
ANSWER: Free rotation around a double bond is not possible because the doubly bonded carbons are bonded by a sigma and a pi bond. For free rotation to occur, pi bond must break. This requires too much energy. That’s why, there is no free rotation around a double bond, and cis-trans isomerism becomes possible in alkenes.
On the other hand, free rotation around a single bond is possible because single bond consists of only sigma bond which results from the linear overlap of the orbitals. For free rotation to occur, there is no need of the breaking of sigma bond. That’s why, cis-trans isomerism in not possible in alkanes.

